Development of a pseudo TPUS probe to improve consistency of patient positioning during MRI.
Eric Pei Ping Pang,
Singapore
PO-1829
Abstract
Development of a pseudo TPUS probe to improve consistency of patient positioning during MRI.
Authors: Eric Pei Ping Pang1, Yong Kang Lim2, Keith Swee Kok Heng2, Chin Hui Tan3, Yufeng, Andy Lin3, Jason Wei Siang Chan1, Gee Keng Low1, Jeffrey Kit Loong Tuan4
1National Cancer Centre Singapore, Division of Radiation Oncology, Singapore, Singapore; 2SingHealth Duke-NUS, Institute for Patient Safety and Quality, Singapore, Singapore; 3National Cancer Centre Singapore, Department of Oncologic Imaging, Singapore, Singapore; 4National Cancer Centre Singapore, Division of Radiation Oncology, Singapore, Singapore
Show Affiliations
Hide Affiliations
Purpose or Objective
Locally, patients undergoing prostate radiotherapy are monitored for prostate displacement using an autoscan 4D transperineal ultrasound (TPUS) probe during treatment. However, the existing TPUS probe and autoscan probe kit (ASPK) are not MRI-compatible. Hence, uncertainties due to variations in patient position and delineation of the prostate gland based on CT/MRI fusion may be introduced during treatment planning. This project aims to develop a MRI-compatible pseudo
TPUS probe and ASPK to reproduce patient position during MRI to facilitate image fusion and prostate gland contouring during treatment
planning.
Material and Methods
The fabricated MRI-safe TPUS probe and ASPK were designed and produced in
components with certain parts required to provide mechanical functions for angulation and height adjustments with similar accuracy of the existing probe used during treatment. Major components were modelled separately for producing parts using MRI-safe materials and assembly. The first prototype was printed as a singular part in both FDM and PolyJet. Selected materials must not introduce any magnetic field interference or contra-indicate MRI procedures (i.e. must not get heated up by magnetic waves). Selected manufacturing process had to consider production of parts that can ensure the rigidity and accuracy of the structure to
withstand pressure on the probe at the transperineal region (i.e. Computerized Numerical Control (CNC) machined
vs 3D printing). CNC process was eventually selected as 3D-printed parts tend to create
material warpage that reduces the accuracy of the design and the material strength required.
Results
Materials that passed both image
test and metal detector test: PVC, PP, ABS, Wood Meranti, Polycarbonate, with Aluminium
Composite failing both image and metal detector test. Acrylonitrile Butadiene Styrene (ABS) was selected as the
MRI-safe material for development of the prototype due to its durability, rigidity, strength, corrosion and
impact-resistant. A test scan was performed on the MRI
scanner and found to be safe and artefact-free for target used cases. (Figure
1). Two rounds of prototyping were conducted to arrive at the final product
(Figure 2).
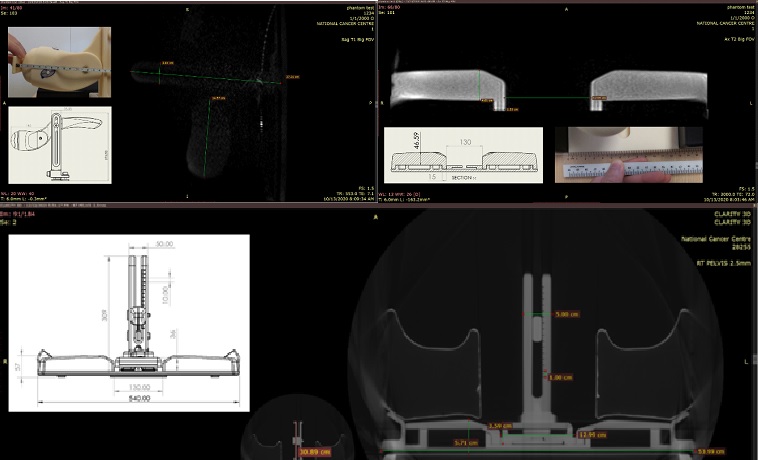
Figure 1: Test scans images
from the MRI Scanner on the final design probe and ASPK setup
(Material passed the image test and dimension accuracy was achieved).

Figure
2: Final CNC machined prototype of the MRI-safe probe and ASPK (with commercial
knee rest).
Conclusion
Final CNC-machined MRI-safe
probe and ASPK was developed for subsequent TPUS prostate patients requiring CT/MRI fusion during radiotherapy treatment
planning. Moving forward, the effectiveness of this gadget on the reproducibility of patient position during MRI to facilitate image fusion for contouring of the prostate gland will be assessed in this novel
clinical workflow for prostate cancer patients. Additionally, the gadget can also be used for patient education to improve compliance during setup.