Metabolic background affects radiation response more than metabolic therapy, in GBM models.
PO-1825
Abstract
Metabolic background affects radiation response more than metabolic therapy, in GBM models.
Authors: Orit Furman1, Keren Porper1, Yael Shpatz1, Yaacov Lawrence1, Leor Zach1
1Sheba Medical Center, Radiation Oncology, Ramat Gan, Israel
Show Affiliations
Hide Affiliations
Purpose or Objective
Animal brain-tumor models have
demonstrated a synergistic interaction between radiation therapy and a ketogenic
diet (KD). Sheba Medical Center single-institution phase I clinical trial was
performed to assess tolerability and feasibility of ketogenic diet (KD)
combined with radiotherapy in gliomas (Porper et al. 2021). In this clinical
trial, Radiotherapy was either 60 or 35 Gy over 6 or 2 weeks, for newly
diagnosed (n=6) and recurrent (n=7) gliomas, respectively. The dietary
intervention consisted of a Modified Atkins Diet (ModAD) supplemented with high-dose
or low-dose metformin with the goal of increasing blood ketone concentration
(beta hydroxybutyrate, bOHB). Factors associated with blood ketone levels were
investigated, and unpublished clinical results indicated a positive correlation
between b-OHB and blood Adiponectin (Adp). Adp is an adipocyte-secreted hormone
that regulates lipid and glucose metabolism, and its receptors are
differentially expressed in gliomas. Previous research indicated Adp protects
fibroblasts, but not prostate or colorectal cancer cells, from radiation-induced death (Kosmacek & Oberley-Deegan 2020).
We used a clonogenic assay to test the hypothesis that Adp and/or b-OHB affect
response of cells in GL261 mouse glioma
model to radiation.
Material and Methods
GL261 cells were seeded in triplicate (600
cells/well in control, 1000 cells/well for treatment) in DMEM medium at either
Low glucose levels (1g/L) or high glucose levels (4.5g/L). Following 24h incubation
with either Adp (1ng/ml), b-OHB (5mM ), both Adp and b-OHB or medium only,
cells were exposed to 0 ,2 or 4 Gray of radiation (Kimtron Polaris Biological
Irradiator). Cells were incubated to form colonies for 2 weeks, after which
cells were fixed, stained with crystal violet (0.5% v/w) and counted under a
microscope. A colony was defined as consisting of at least 50 cells.
Results
Adiponectin or ketone
treatments did not significantly affect the fraction of surviving cells, while
the level of glucose in the medium affected both plating efficiency (PE) and
order of magnitude of surviving fraction of cells. PE in High Glucose ranged
between 45-65% while Low Glucose PE was 18-25%. Under High Glucose conditions,
more than 10% of cells irradiated with 4 Gray created colonies, regardless of
drug treatment, while in Low Glucose only 1% of cells created colonies
regardless of drug treatment. 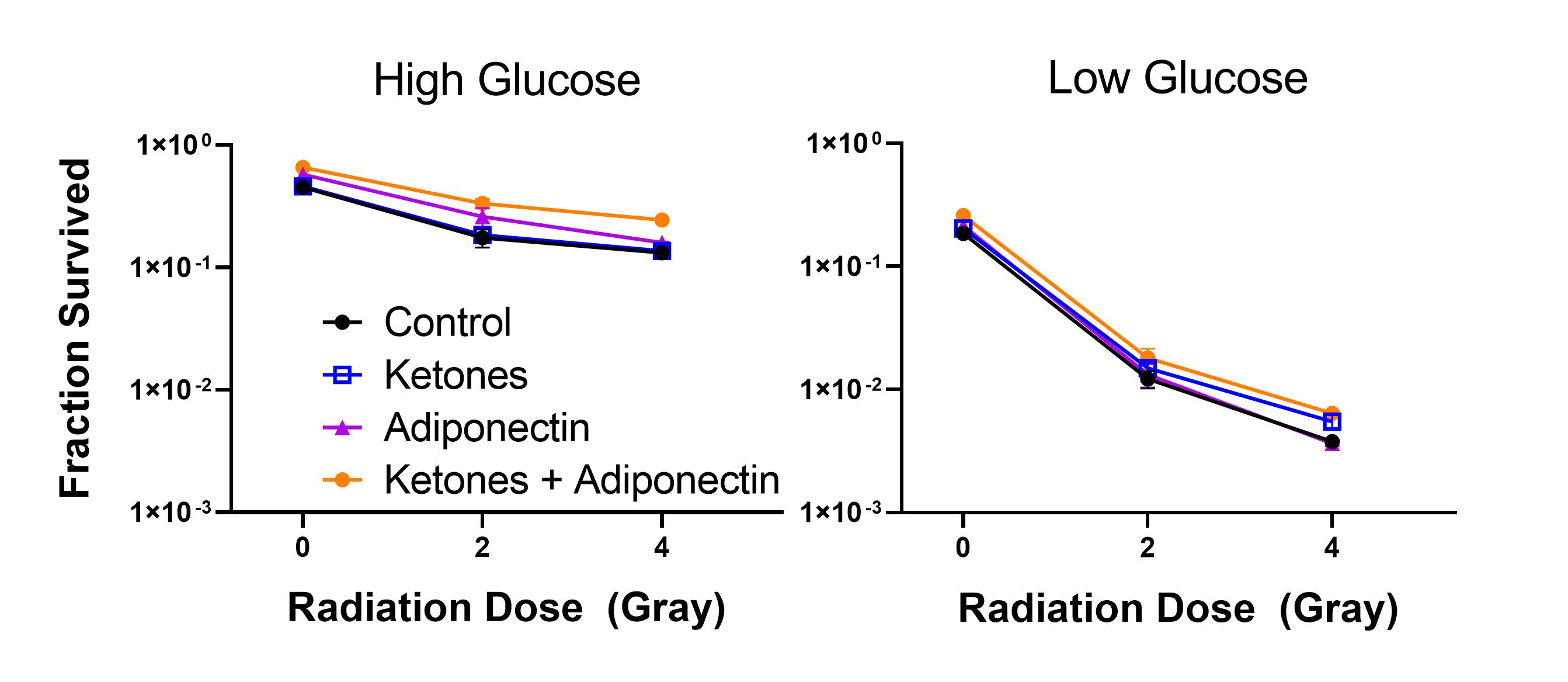
Conclusion
Metabolic background was more
effective in predicting response to radiation than metabolic treatments. While
it is known that high blood glucose levels predispose cancer development, we
show in a mouse model that high glucose levels also limit the effects of
radiation treatment regardless of Adp or b-OHB levels. If replicated in human
cell culture models and animal in vivo models, this work has implications for
development of efficient metabolic treatments to synergize radiation therapy
for cancer.