Simulated liver implantation (SLIM): Towards fully robotic LDR brachytherapy for liver tumors
Philipp Aumüller,
Germany
PO-1803
Abstract
Simulated liver implantation (SLIM): Towards fully robotic LDR brachytherapy for liver tumors
Authors: Philipp Aumüller1, Andreas Rothfuss2, Michael Ehmann3, Frank A Giordano4, Sven Clausen3
1University Medical Centre Mannheim, University Heidelberg, Germany., Department of Radiation Oncology, Mannheim, Germany; 2Fraunhofer , IPA, Mannheim, Germany; 3University Medical Centre Mannheim, University Heidelberg, Germany, Department of Radiation Oncology, Mannheim, Germany; 4University Hospital Bonn, Department of Radiation Oncology, Bonn, Germany
Show Affiliations
Hide Affiliations
Purpose or Objective
Low dose rate
brachytherapy (LDR-BT) may be a minimally invasive and highly effective therapy
to treat liver tumors, especially using (semi- or fully-) robotic implantation.
We recently introduced an inverse treatment planning system for robotic LDR-BT
with path planning independent of a rigid template to guide needles to the
target volume [1]. The spatial
registration of each implanted seed with direct feedback to the planning system
during the implantation procedure enables adapting the remaining needle and
seed positions to correct systematic and statistical dose errors introduced to
the original treatment. We here show results of
a simulated annealing adaption (SAA) algorithm that modifies subsequent needle
positions online to optimize the original treatment planning objectives in a
simulation study in the setting of LDR-BT for liver tumors.
Material and Methods
We performed simulated liver implantations (SLIMs) by
sequentially adding values of a normally distributed deviation based on the
uncertainty of the placement accuracy (PA) to the tip coordinate of each needle that
is about to be implanted and recalculating the dose distribution resulting from
the already implanted and the remaining pre-planned needle and seed positions.
The SAA algorithm uses
the MATLAB function simulannealbnd (The MathWorks-R2021a) to vary the remaining needle tips after each simulated
needle implantation in the range of ±1.5mm and to minimize the objective function designed
to spare organ at risks (OARs), increase the V100 and decrease the V200. All
plan parameters are optimized to remain near the pre-treatment values except a
restrictive dose constraint to 1%
of the aorta
(D1). Ten SLIMs of one treatment plan for a liver tumor in
an abdominal phantom with SAA or no SAA (NSAA) with a PA
of ±1mm and ±3mm are compared.
Results
The SLIM V100 were for a PA of ±1mm SAA (98.5±0.3)% , NSAA (99.0±0.4)% and for ±3mm (97.0±1.2)% and (96.0±1.3)% respectively. SAA reduces the D1(aorta) and maintains other treatment aims. A higher PA of ±1mm against ±3mm results in a
higher plan robustness due to smaller standard deviations of the planning
parameters.
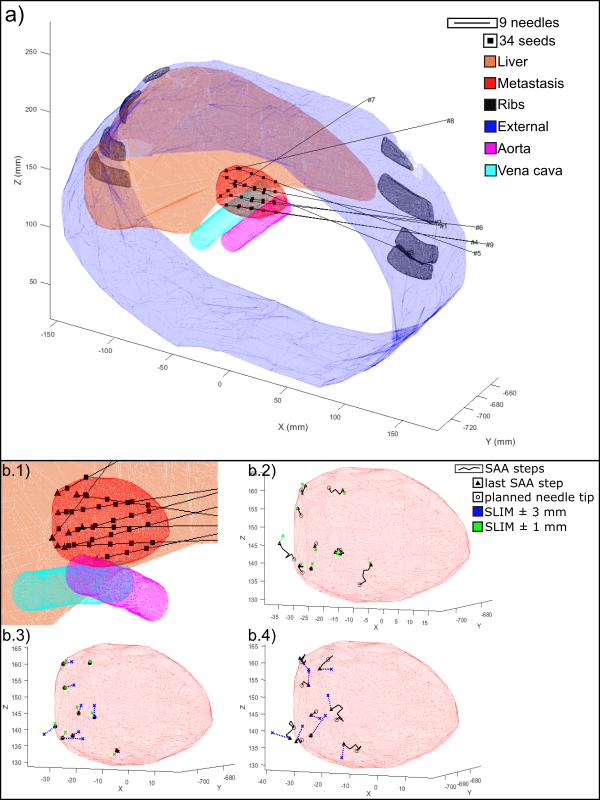
Fig. 1 a): Abdominal phantom
with the pre-treatment plan. b.1) Close view. b.2) SLIM with SAA and PA±1mm. b.3) NSAA±{1;3}mm. b.4) SAA±3mm.
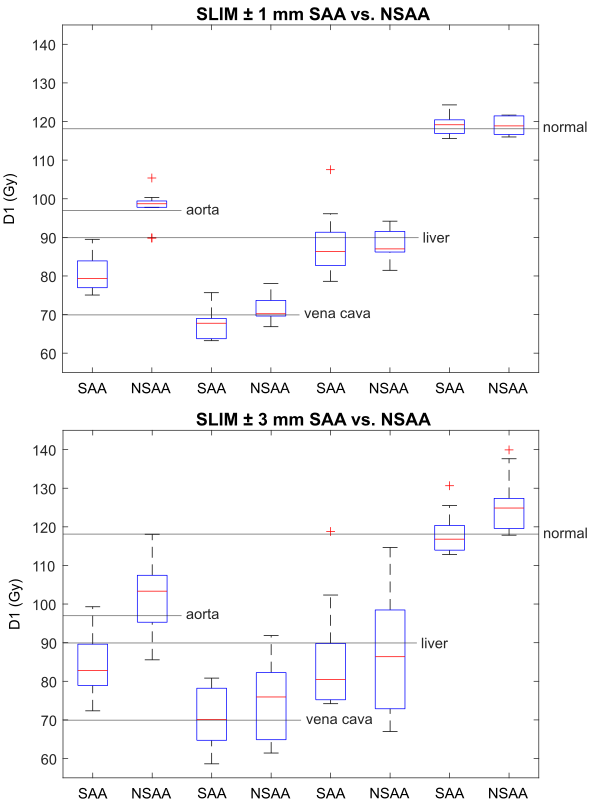
Fig. 2: OAR
D1 doses for SLIM with PA of ±{1;3}mm with SAA or
NSAA.
Conclusion
The SAA algorithm is able to correct the remaining needles to achieve
better planning outcomes in terms of plan robustness and sparing of OARs. Its
implementation into the workflow is the last missing piece of assembling a
fully robotic LRD-BT, which might now enable safe and rapid minimally invasive
therapy of liver tumors.
[1] P. Aumüller, A. Rothfuss, M. Polednik, Y. Abo-Madyan, M. Ehmann,
F.A. Giordano, S. Clausen, Multiple direction needle-path planning and inverse
dose optimization for robotic low-dose rate brachytherapy, Zeitschrift für Medizinische
Physik. (2021). https://doi.org/https://doi.org/10.1016/j.zemedi.2021.06.003.