Comparison of different strategies for deformable dose accumulation in prostate cancer radiotherapy
PO-1712
Abstract
Comparison of different strategies for deformable dose accumulation in prostate cancer radiotherapy
Authors: Martina Murr1, Daniel Wegener2, Marcel Nachbar1, Arndt-Christian Müller3, Daniel Zips2, Daniela Thorwarth1
1University of Tübingen, Section for Biomedical Physics, Department of Radiation Oncology, Tübingen, Germany; 2University Hospital and Medical Faculty, University of Tübingen, Department of Radiation Oncology, Tübingen, Germany; 3RKH Kliniken, Clinic of Radiation Oncology and Radiation Therapy, Ludwigsburg, Germany
Show Affiliations
Hide Affiliations
Purpose or Objective
Online MR-guided radiotherapy (MRgRT) enables optimal plan adaptation with respect to the patient’s daily anatomy and thus allowing maximum target coverage and organ at risk (OAR) sparing. To date methods for deformable dose accumulation (DDA) are lacking, which would be a major prerequisite for the development of accurate dose-response models and precise clinical dose constraints. The aim of this study was to develop and implement different strategies of DDA and compare them to a fraction-based dose summation (DS).
Material and Methods
A total of 5 prostate cancer (PC) patients treated at a 1.5T MR-Linac (20 x 3 Gy) were included into this retrospective study.
Two different DDA strategies for online adaptive MRgRT were compared. First, retrospective (re) DDA was carried out, where all adaptive MRgRT fractions were deformably registered to the MRI of the first fraction (fx01). The second strategy consisted of a sequential (seq) DDA, applying deformable registration and subsequent dose warping of one data set to that of the previous fraction, e.g. fx03 to fx04. For automatic OAR contouring (MR deep learning model) based on T2w MRI and deformable image registration Monaco ADMIRE (Elekta) was used, whereas DDA and DS were realized via 3Dslicer (open source software). Clinically relevant dose criteria for PC (cf. table 1) were assessed for prostate, bladder, and rectum. The resulting dose distributions were compared to a fraction-based summation of these criteria mimicking the current MR-Linac workflow where no DDA is available.
Results
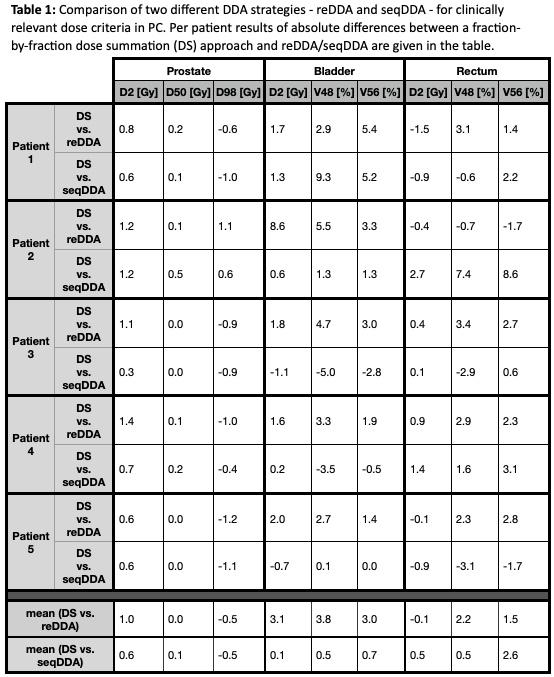
Table 1 summarizes the absolute differences between the dosimetric criterial obtained with the two different DDA strategies and a fraction-wise dose summation approach. Mean absolute differences between DS and reDDA for prostate D2%, D50% and D98% were 1.0 Gy, 0.0 Gy and -0.5 Gy, respectively. In contrast, differences of bladder D2%, V48Gy and V56Gy resulted in 3.1 Gy, 3.8% and 3.0%, as well as for rectum -0.1 Gy, 2.2% and 1.5%. The comparison of seqDDA and DS yielded mean absolute differences of D2 = 0.6 Gy, D50 = 0.1 Gy and D98 = -0.5 Gy, for bladder D2 = 0.1 Gy, V48 = 0.5% and V56 = 0.7%, and for rectum D2 = 0.5 Gy, V48 = 0.5% and V56 = 2.6%, respectively.
Conclusion
In this study, we showed first results of two different DDA strategies for adaptive MRgRT of PC. Mean differences between fraction-wise DS and DDA approaches were only small, whereas, for individual patients, large differences were observed especially for maximum OAR dose tolerances which could result in a detrimental impact on quality of life. Furthermore, the two dose accumulation strategies show a variation compared to each other, especially in OAR. A further investigation with a larger number of patients is required before DDA strategies can be used in clinical practice to steer online adaptive MRgRT.