SBRT-responsible
physicists at the sites offering stereotactic radiosurgery (SRS) or SBRT at the
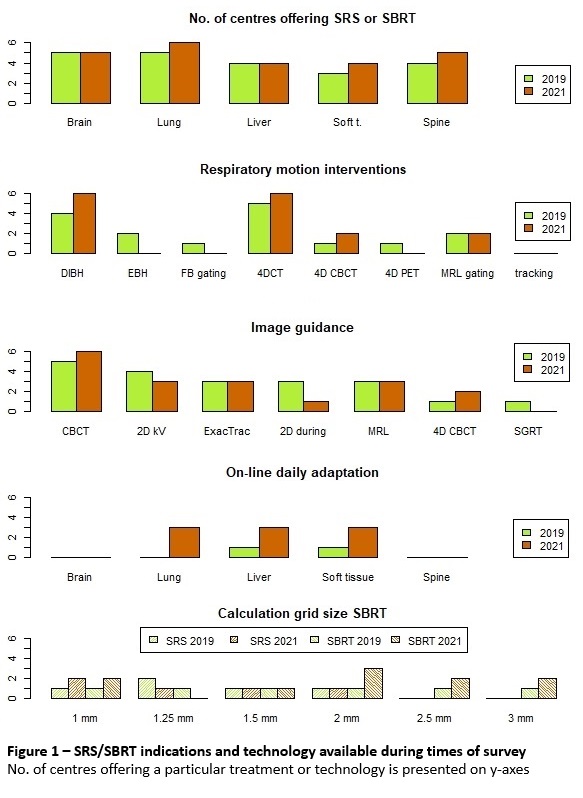
time, filled out the survey. Figure 1 shows availability of SRS/SBRT
indications and technology. Treatment planning systems differed, but all
applied advanced dose calculation algorithms (type B or better).
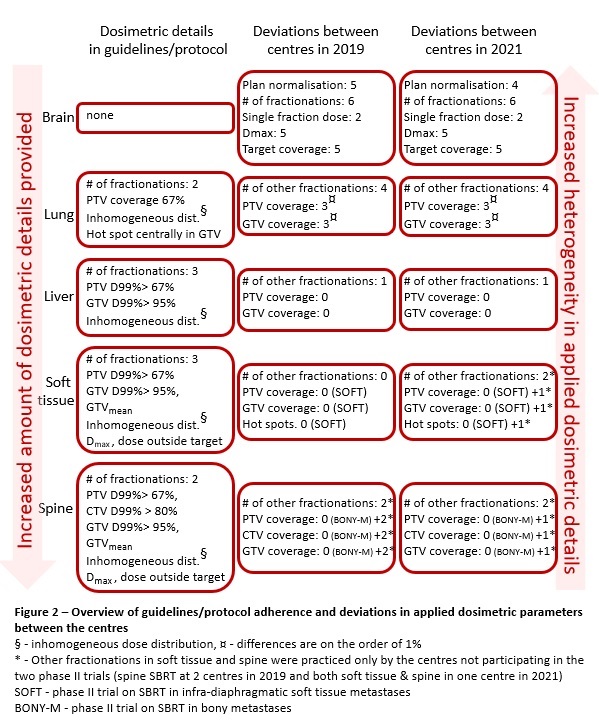
Brain SRS
heterogeneity was high: 6 different fractionations, dose prescription to either
GTV (4 centres) or PTV (1 centre), highest single fraction dose of 18Gy (2
centres) or 20Gy (3 centres) and 5 different target coverage objectives. National
SRS guidelines don’t provide any dosimetric details.
Multiple lung SBRT
fractionations were applied, not all described in the guidelines. PTV coverage of
67%, simply specified in the guidelines, was clinically applied as PTV D99% or
D98%. GTV coverage, not specified in the guidelines, was applied in three
different ways.
Liver SBRT
fractionations from guidelines and SOFT protocol (with one additional
fractionation at one institution) were applied. Clearly prescribed GTV and PTV
coverage from the guidelines was applied at all centres, with two having an
additional GTV coverage aim.
The dosimetric
parameters did not change in the 2-year period for brain, lung and liver SBRT.
For soft tissue SBRT, the
three centres participating in the trial used target coverage per trial
specification, which differed from the fourth centre, first starting with soft
tissue SBRT in 2021.
In spine SBRT, target
coverage consistency improved between the centres in 2021, with four of them
participating in the phase II trial in 2021 and only two at the time of the
first survey.
An overview of guidelines/protocol
adherence and deviations in applied dosimetric parameters between the centres is
shown in Figure 2.