Feasibility of utilizing Spot-Scanning Proton Arc (SPArc) therapy for whole Lung Irradiation
PO-1651
Abstract
Feasibility of utilizing Spot-Scanning Proton Arc (SPArc) therapy for whole Lung Irradiation
Authors: Lewei Zhao1, Gang Liu2, An Qin3, Di Yan1, Craig Stevens3, Xiaoqiang Li3, Rohan Deraniyagala1, Xuanfeng Ding1
1Beaumont Health System, Radiation Oncology, Royal Oak, USA; 2Huazhong University of Science and Technology, Tongji Medical College, Wuhan, China; 3Beaumont Health System, Radiation Oncology , Royal Oak, USA
Show Affiliations
Hide Affiliations
Purpose or Objective
We reported the first feasibility study of utilizing spot-scanning arc therapy (SPArc) whole lung irradiation for a pediatric patient to reduce cardiac toxicity.
Material and Methods
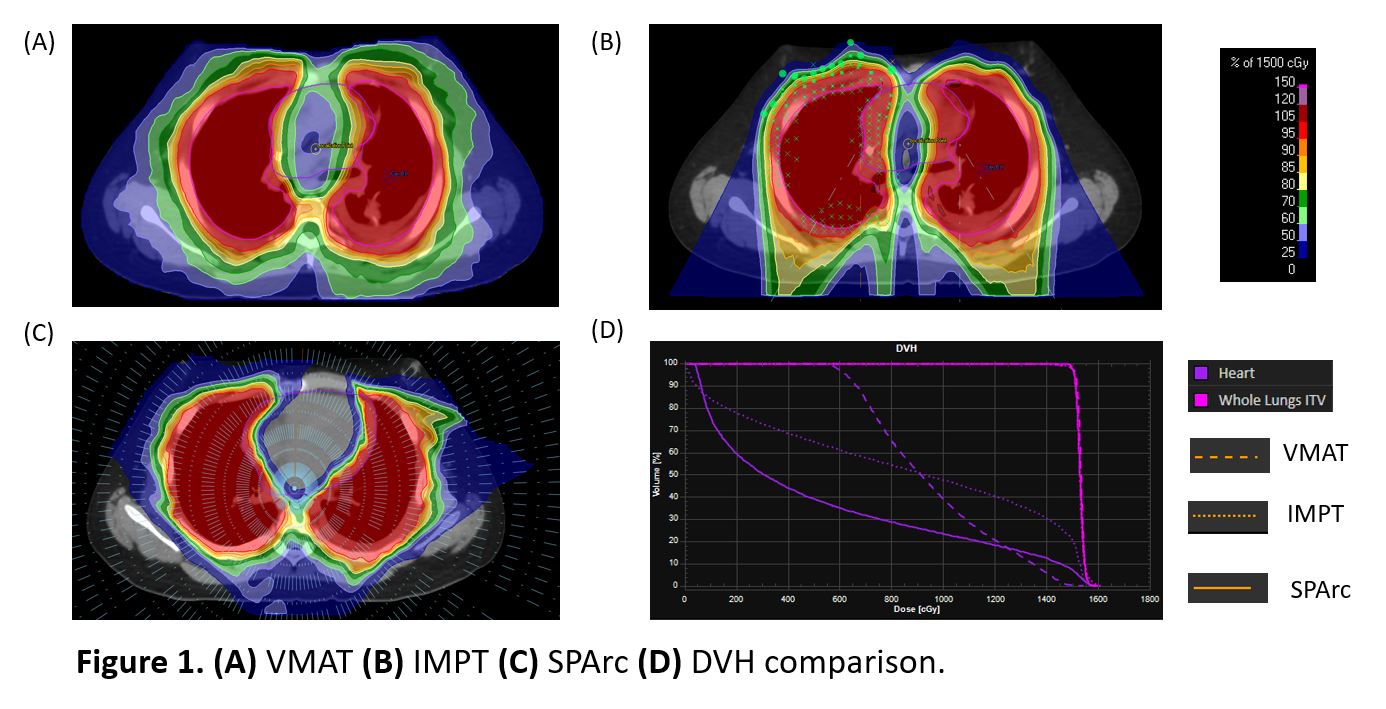
A 13 year-old patient with metastatic Ewing sarcoma treated with Volumetric Modulated Arc Therapy (VMAT) in our institution was selected (Figure 1A). The clinical VMAT plan used three 360-deg arcs with 6 MV based on the Elekta HD. Intensity Modulated Proton Therapy (IMPT) plan was generated using 2 iso and 4 treatment fields (Figure 1B) based on the single field optimization approach in a clinical treatment planning system (TPS) (RayStation version 6). SPArc plan was generated in the same TPS through in-house scripting using a full 360-deg arc trajectory with 2.5-deg frequency via a single isocenter (Figure 1C). The Proton beam model was based on a synchrocyclotron system with 70-227 MeV energy. Both IMPT and SPArc plans applied the same robust optimization parameters (±5mm setup and ±3.5% range uncertainties). The prescription was 1500cGy in 10 fractions. VMAT, IMPT, and SPArc plans were normalized to at least 98% of the ITV receiving the prescribed dose. Heart dose-volume histograms (DVHs) and integral body dose were evaluated among the VMAT, IMPT, and SPArc plans. VMAT, IMPT, and SPArc plans' delivery efficiency were simulated based on the machine delivery sequence models. The VMAT delivery time was acquired from the LINAC machine logfile.
Results
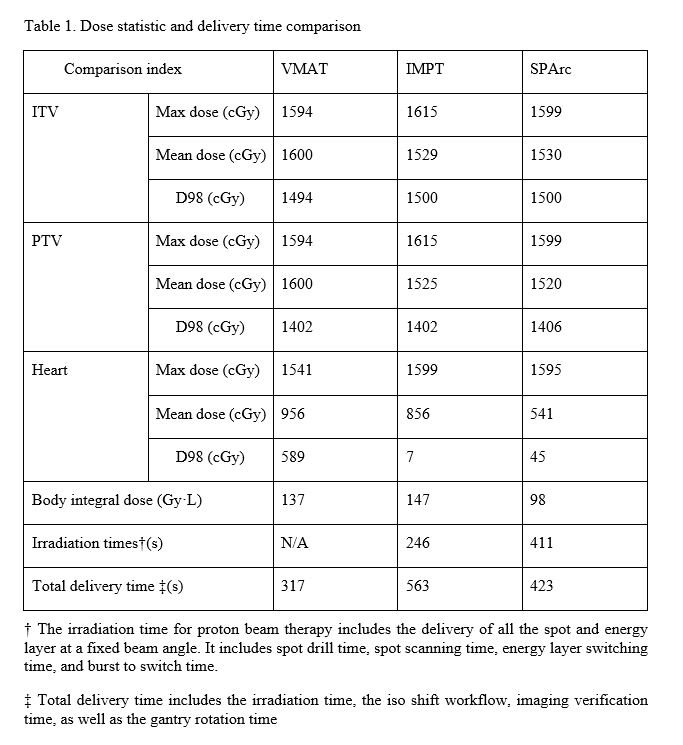
The SPArc plan significantly spared the dose delivered to the healthy tissue, compared to the IMPT and VMAT plan, while providing similar coverage to the clinical target volumes (Table 1): the mean dose to the heart was 541 cGy in SPArc, compared with 856 cGy in the IMPT, and 956 cGy in the VMAT, respectively. The integral body dose was 137 Gy·L in VMAT,147Gy·L in IMPT, and 98 Gy·L in SPArc ( Figure 1). The LINAC logfile showed that VMAT took 317s to deliver all three arcs. Based on a fixed gantry machine-specific delivery sequence simulation, it costed the IMPT plan 246s and SPArc plan 411s to irradiate all the spots and energy layers. Considering the additional parameters, such as the gantry mechanical motion and iso-center shift, the two-iso IMPT plan would require additional 5 mins for couch shift and imaging validation which makes the total treatment time around 563s. In contrast, SPArc does not require any iso shift. The simulation result from the DynamicARC® delivery showed the total treatment delivery 423s which is only 12s extra was used in the control point connection from the gantry mechanical limitations. (Table 1). Thus, SPArc was able to reduce more than 25% of the total treatment delivery time compared to IMPT.
Conclusion
Our SPArc technique showed a significant dosimetric benefit in cardiac and integral dose sparing compared to VMAT and IMPT in the whole lung irradiation. Additionally, SPArc could simplify the clinical workflow with a single iso and improve the treatment delivery efficiency through the arc trajectory compared to the IMPT.