Novel concept for patient-specific immobilization using generative design and additive manufacturing
PO-1635
Abstract
Novel concept for patient-specific immobilization using generative design and additive manufacturing
Authors: Bertrand Dewit1, Tom Depuydt2,1
1UZ Leuven, Radiation Oncology, Leuven, Belgium; 2KU Leuven, Oncology, 3000, Belgium
Show Affiliations
Hide Affiliations
Purpose or Objective
Thermoplastic masks are currently the
standard in RT immobilization. These can however be uncomfortable due to
shrinkage, induce claustrophobia and can limit beam angle selection in
treatment plans. We therefore propose a novel concept for more comfortable
patient-specific and plan-optimized head and neck immobilization devices using
the full potential of additive manufacturing (AM) and Computer-Aided Design
(CAD).
Material and Methods
To design
the immobilization, CT data of a head and neck patient was retrospectively
collected. The body contour was segmented from the head up to the shoulders in
Mimics® (Materialise) and exported as a 3D mesh. All subsequent CAD was
performed in fusion360® (Autodesk). First, the body mesh was converted into a
solid modelling boundary representation and positioned on a concept model of
the envisioned treatment table, including two attachment sites for the immobilization.
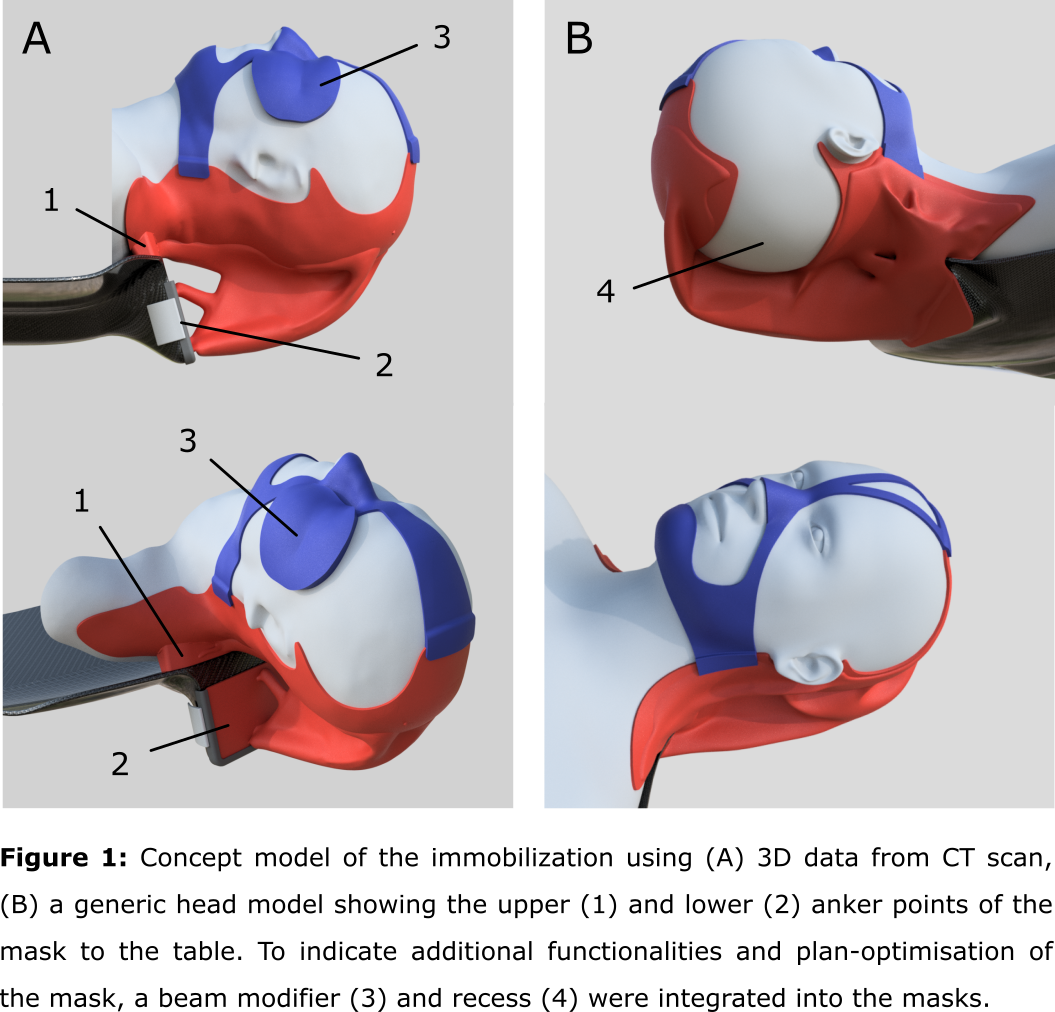
Next, a two-part mask was designed around the patient’s body. The lower shell
includes the shoulders and a cranial stop and the upper part the nose bridge
and the chin (figure 1). Using AI assisted generative design (GD) the
connection between the lower shell and the table attachments were generated
using a downward load case of 35 N to represent an adult human head and HP PA12
nylon as the printing material of choice. Regions to be avoided such as a
treatment machine bore or nozzle can be taken into account in the GD process. A
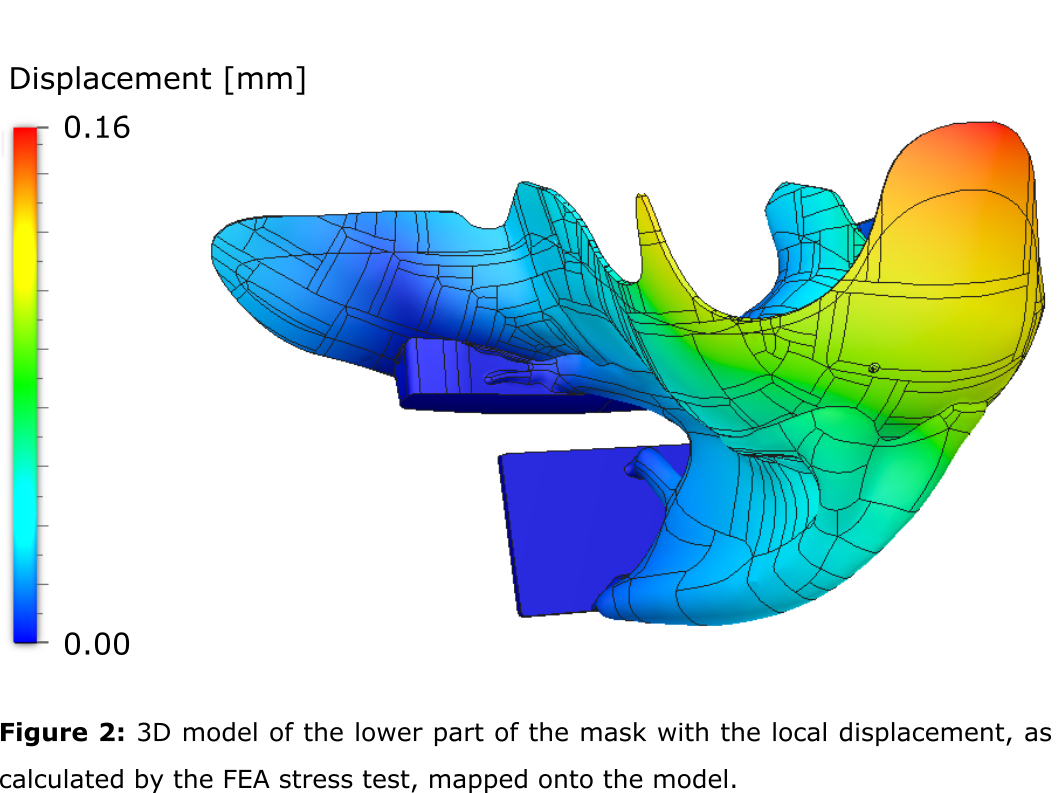
Finite Element Analysis (FEA) static stress test was performed to calculate the
factor of safety (FoS) and expected displacement.
Results
The proposed workflow allowed for the creation of a
patient-specific open face mask starting from CT data. We were able to integrate
additional functionalities to the immobilization structure such as a beam
modifier (bolus/range shifter) and in-vivo dosimetry which can ensure accurate
placement of these devices with respect to the patient anatomy. The
immobilization device could also be customized to the treatment plan, by avoiding
the beam having to pass through material or structural edges which can disturb
the intended dose distribution in the patient (figure 1). This can ultimately
allow more flexibility in the beam angle selection, potentially resulting in an
improved treatment plan quality. The static stress test indicated a realized FoS
of 9.6, indicating that further optimisation of the support could reduce the
use of material and production cost given that a safety factor of 2-3 should
suffice. A maximal displacement of 0.16 mm was indicated (figure 2).
Conclusion
The
realised FoS and maximal displacement given by the FEA indicates that our novel
immobilization using GD and AM is a viable concept. We therefore conclude that the
proposed workflow shows to be a promising new concept for patient immobilization.
Future work would include investigating the use of surface scanning and extensive
testing of the stability, reproducibility and comfortability of the immobilization
devices using phantoms and eventually patients.