Proton therapy dose distributions around cardiac implant leads measured with 3D dosimeters and films
PO-1571
Abstract
Proton therapy dose distributions around cardiac implant leads measured with 3D dosimeters and films
Authors: Lia Valdetaro1, Line Bjerregaard Stick2, Mateusz Krzysztof Sitarz3, Ludvig Paul Muren1, Peter Balling4, Peter Sandegaard Skyt3, Jørgen Breede Baltzer Petersen5, Maria Fulgsang Jensen3
1Aarhus University, Department of Clinical Medicine, Danish Center for Particle Therapy, Aarhus, Denmark; 2Aarhus University Hospital , Danish Center for Particle Therapy, Aarhus, Denmark; 3Aarhus University Hospital, Danish Center for Particle Therapy, Aarhus, Denmark; 4Aarhus University, Department of Physics and Astronomy, Interdisciplinary Nanoscience Center, Aarhus, Denmark; 5Aarhus University Hospital, Medical Physics, Department of Oncology, Aarhus, Denmark
Show Affiliations
Hide Affiliations
Purpose or Objective
Due to their proximity to lymph nodes,
cardiac implantable electronic device leads can present a challenge to target
coverage in proton therapy of breast cancer patients. Moreover, shortcomings in
treatment planning dose calculations when modelling the interaction of proton
beams with metal components can result in greater uncertainty in the
delivered dose. The aim of this study was therefore to dosimetrically
investigate the dose degradation caused by two types of leads with 3D
radiochromic dosimeters and 2D gafchromic films.
Material and Methods
Radiochromic dosimeters
(5×5×7 cm3) were fabricated from
silicone, curing agent, chloroform and leucomalachite green (LMG). Before
curing, a cylindrical insert with a 3 mm diameter was placed in the centre of
each dosimeter, to fit the lead during irradiation. Leads of two different
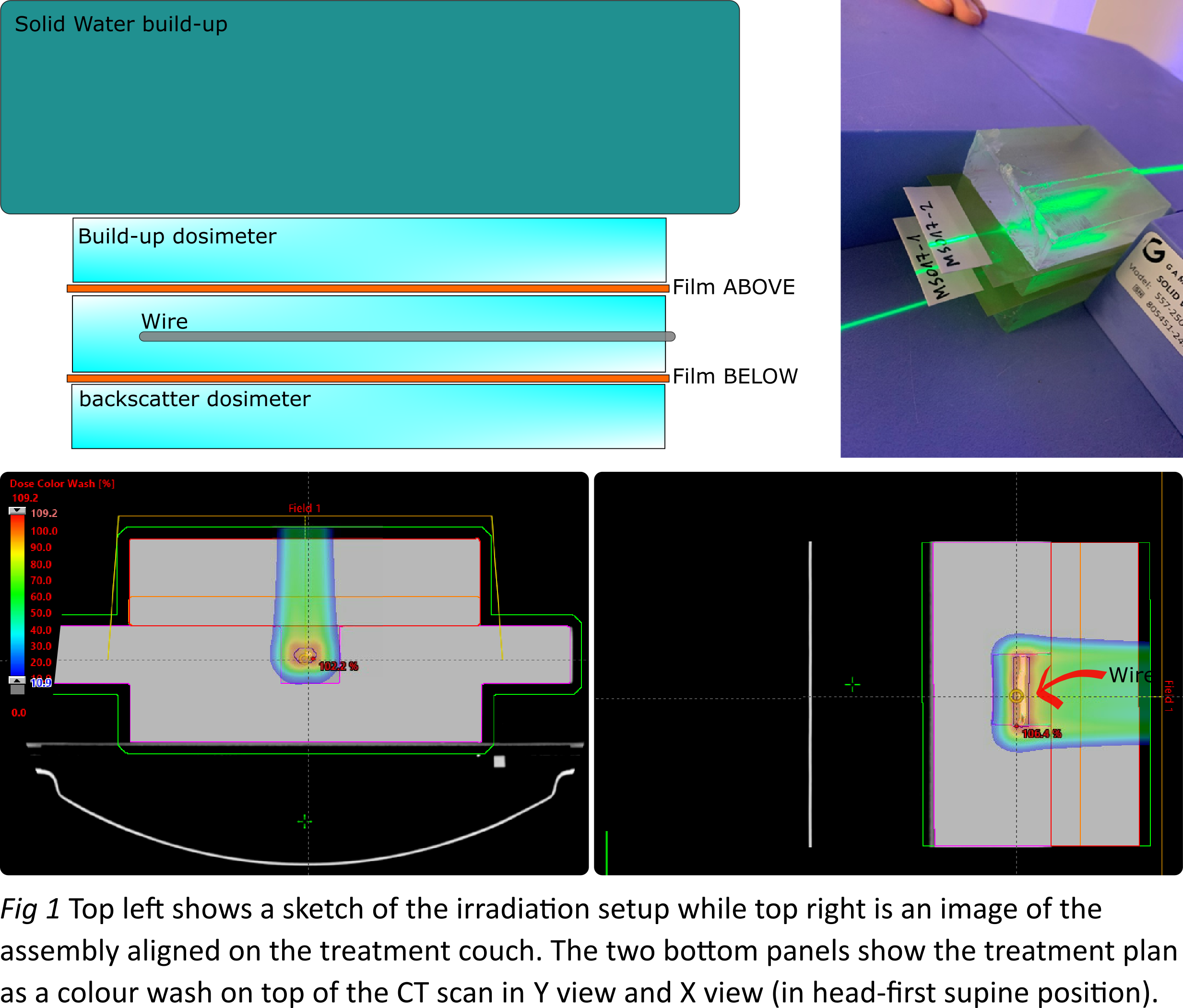
widths (Ø1.6 mm and Ø2.2 mm) were used. EBT3 gafchromic films (5 × 7 cm2) were placed 1 cm above and 1 cm below
the leads (see Fig 1). The entire setup was CT-scanned and imported to Eclipse
(Varian Medical Systems) where two spot-scanning proton therapy plans were
prepared using 2.5 or 7.5 cm thickness solid water (SW) build-ups, such that
the leads were positioned at the spread-out Bragg peak. A 5 cm range shifter
(water equivalent thickness of 5.7 cm) was used in both plans that also
delivered 6 fractions of 2 Gy (RBE dose) to the region containing the lead.
Following our previously established dosimetry protocols, gafchromic films were
scanned 24 hours before and after irradiation with an Epson Expression Pro
scanner while the radiochromic dosimeters were scanned 2 hours before and after
irradiation using an optical CT scanner (Modus Medical) with 1000 projections
over a 360° rotation. Subsequent data reconstruction for the 3D
dosimeters was performed with 0.5 mm3 voxel
size.
Results
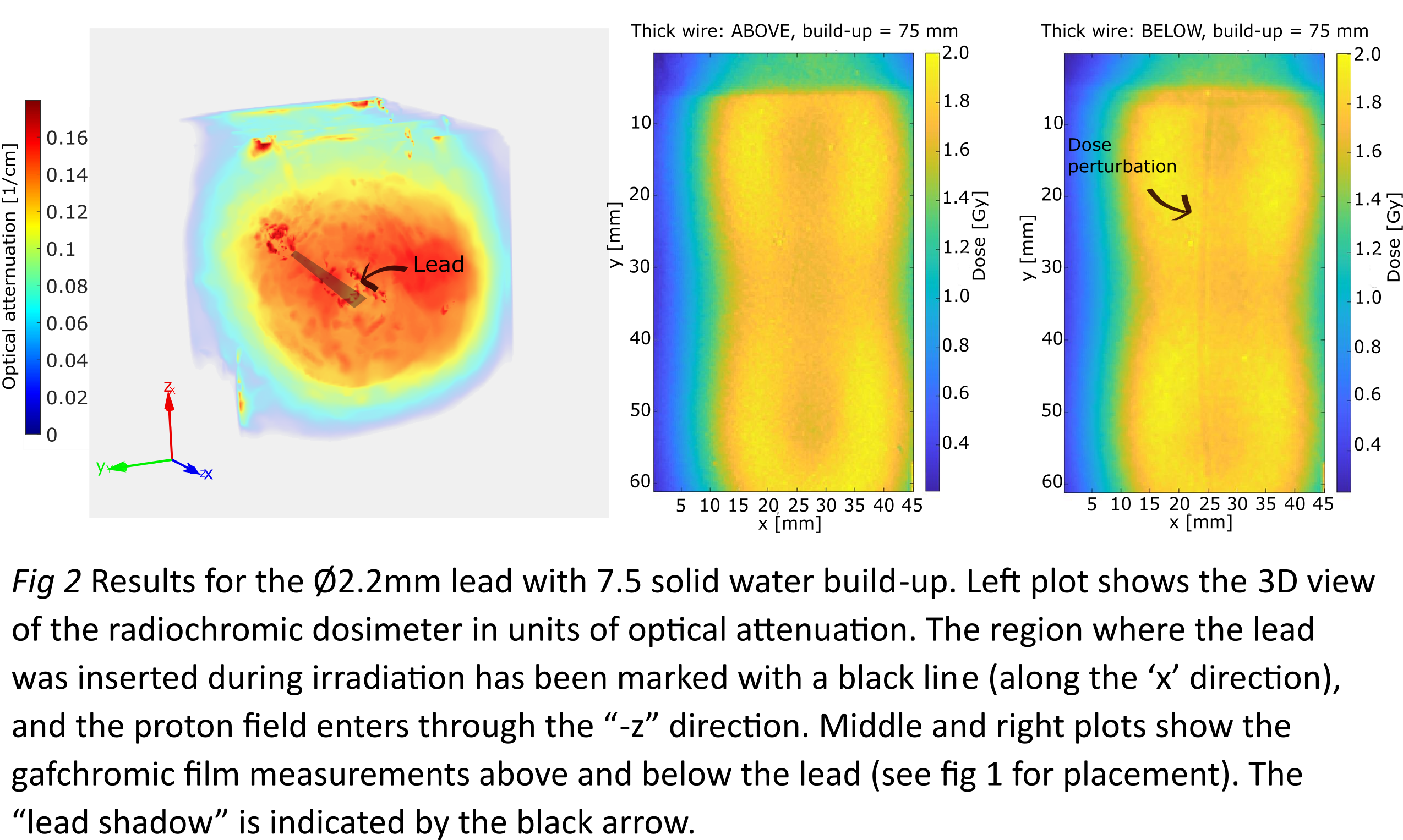
With respect to dose degradations, the
largest under dosage (12%) was observed for the thick lead (Ø2.2mm) and 7.5 cm
SW build-up, while the smallest under dosage (6%) was observed for the Ø1.6mm
lead and 2.5 cm SW build up. Under dosage was localised behind the leads in the
beam direction and had approximately the same width and shape as the leads
themselves. No overdosage due to backscatter radiation could be observed with
neither films nor radiochromic dosimeters.

Conclusion
Both lead
thicknesses caused dose degradations,
with the smallest shadowing effect measured for the thin lead (Ø1.6 mm).
Since underdosage was localised in the
beam direction downstream of the leads, its effect could be minimized in patient plans by using more than one
field at different incidence angles.