Combining multicriteria optimization with knowledge-based planning in brain tumor radiotherapy
Siete Koch,
The Netherlands
PO-1520
Abstract
Combining multicriteria optimization with knowledge-based planning in brain tumor radiotherapy
Authors: Siete Koch1, Coen Stevelink1, Anand Bhawanie1, Anja Jonkman1
1Medisch Spectrum Twente, Radiotherapy, Enschede, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Treatment
planning for a brain tumor can be challenging due to the close proximity of
numerous critical structures. Our previous study demonstrated a clear benefit of using knowledge-based objectives to drive the plan optimization
process. As a next step, the current study explores the potential of multicriteria optimization (MCO) to reduce
the dose to critical structures even further.
Material and Methods
Retrospective
re-optimization
was performed by a single planner using the RapidPlan and MCO tools of Eclipse 15.6 (Varian, Palo Alto, US). The study population
consisted of 20 patients prescribed 30×2 Gy for
glioblastoma multiforme or meningioma. Target volumes varied in location and
size (mean PTV 298 cc, range 121-491 cc). Up to 14 brain and optic structures per
patient had been contoured (minimum 11), with at least one structure partially
overlapping the PTV. The clinical VMAT setup on a TrueBeam linac was retained
in replanning.
The DVH
estimation model was described previously. For each patient, the line
objectives from RapidPlan were selected for MCO trade-off exploration. MCO
generates a series of optimal plans based on the entered objectives, enabling the planner to navigate to a preferred solution.
RapidPlan
and MCO plans were compared in terms of mean doses to critical structures. A
paired samples t-test was performed per structure type. In addition, PTV doses
were assessed by means of a homogeneity index HI = (Dmax - Dmin)/Dmean.
Results
A net
improvement was consistently achieved with MCO, but the magnitude varied
between patients. In four cases it was possible to reduce the Dmean in each of
the contoured structures. The remaining sixteen cases showed more of a
trade-off with a few structures receiving a higher Dmean. However, this was always
outweighed by a majority of structures receiving a lower Dmean.
For the
entire study population, MCO
reduced the Dmean in 213 out of 267 contoured structures (80%). The remainder received
a higher Dmean (12%) or were unchanged within ±1% (8%).
Separating
by structure type, the overall Dmean statistics were improved for 11 out of the
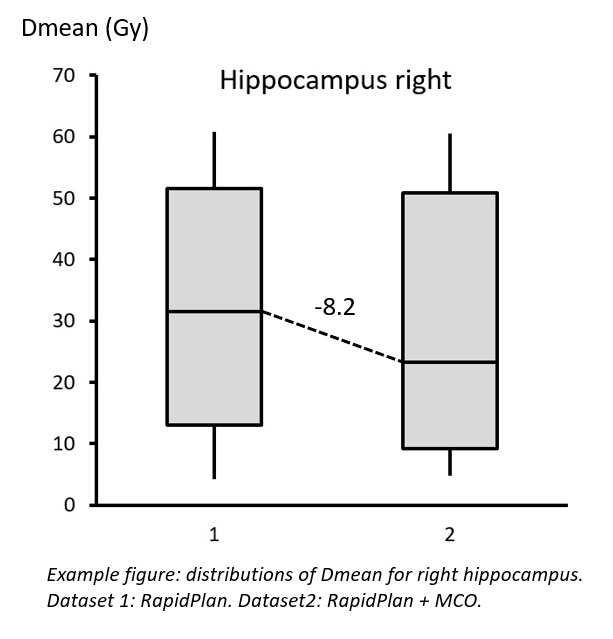
14 structures (p<0.05). The largest difference was observed for the right
hippocampus (median value -8.2 Gy: see figure), followed by the optic chiasm (-5.5 Gy) and
the pituitary (-3.8 Gy). An increase in Dmean resulted only for the left
hippocampus (median value +1.2 Gy).
A trade-off was more evident in the PTV dose
distributions. MCO generally yielded lower minima and higher maxima (within
clinically acceptable limits). The group mean homogeneity index was 0.30±0.08 and 0.35±0.06
before and after replanning, respectively.
Conclusion
Multicriteria optimization offered a considerable
extra benefit in treatment planning for brain tumors. Critical
structures could be spared to a greater extent than with knowledge-based
optimization alone. The main limiting factor is the PTV dose homogeneity. The
combination of MCO with knowledge-based planning is therefore recommended for
brain tumor treatment planning.