Proton therapy for nasopharyngeal cancer: dosimetric and NTCP analysis supporting clinical decision
PO-1509
Abstract
Proton therapy for nasopharyngeal cancer: dosimetric and NTCP analysis supporting clinical decision
Authors: Alessandro Vai1, Silvia Molinelli1, Eleonora Rossi1, Nicola Alessandro Iacovelli2, Giuseppe Magro1, Anna Cavallo2, Emanuele Pignoli2, Tiziana Rancati3, Alfredo Mirandola1, Rossana Ingargiola1, Barbara Vischioni4, Maria Bonora1, Sara Ronchi1, Mario Ciocca1, Ester Orlandi1
1CNAO Foundation, Radiotherapy, Pavia, Italy; 2National Cancer Institute (INT), Radiotherapy, Milan, Italy; 3National Cancer Institute (INT), Prostate Cancer Program, Milan, Italy; 4CNAO Foundation, Radiotherapy, Milan, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
The
aim of the study was to investigate an integrated strategy to quantify
potential benefits in terms of toxicity reduction of intensity-modulated proton
therapy (IMPT) compared to volumetric modulated arc therapy (VMAT) for
nasopharyngeal carcinoma (NPC) patients.
Material and Methods
For
50 consecutive locally advanced NPC patients already treated with definitive
VMAT and chemotherapy, IMPT plans were optimized and compared evaluating target
coverage, homogeneity and conformity indexes (CI), mean and near-to-maximum
doses to organs at risk (OARs). Normal tissue complication probability (NTCP)
differences were calculated using sixteen validated models (ΔNTCPx-p) and
stratified for tumor staging. The patient eligibility for IMPT was assessed using
a model-based selection (MBS) strategy following the results on ΔNTCPx-p for 7/16
models describing the most clinically relevant endpoints. A single ΔNTCPx-p
threshold of 15% to 5% was set on each model, depending on the severity of the considered
complication, or a composite ΔNTCPx-p threshold of 35%. Finally, we developed a
comprehensive toxicity score (CTS), defined as the weighted sum of all 16 ΔNTCPx-p,
where weights follow a clinical rationale.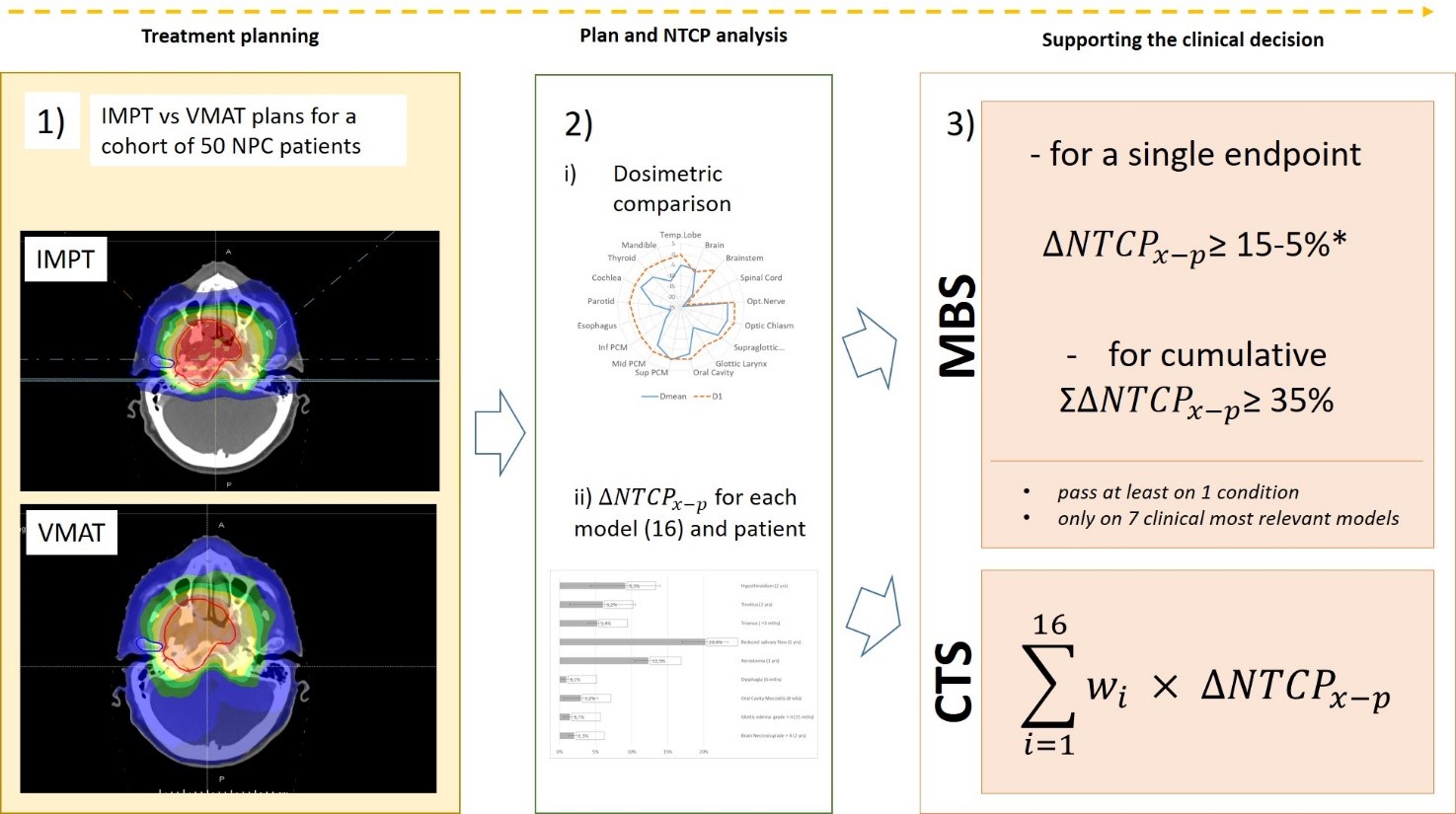
Results
Target dose coverage was guaranteed
for all plans (PTV D99% ≥ 67.5 Gy(RBE)), with IMPT showing
comparable homogeneity and improved conformity (DCI of -10.9%) over VMAT. For OARs, mean dose
deviations were in favor of IMPT (DDmean ≥14% for cord, esophagus, brainstem
and glottic larynx). The risk of toxicity significantly decreased for xerostomia
(-12.5%), brain necrosis (-2.3%), mucositis (-3.2%), tinnitus (-8.6%),
hypothyroidism (-9.3%) and trismus (-5.4.%). Following MBS strategy, forty per cent of the analyzed
patients resulted eligible for proton therapy, with a greater advantage for T3-T4
staging (see Table 1). Significantly different CTS were observed in patients
qualifying for IMPT.
|
|
All pts
|
Tumor staging
|
Nodal involvment
|
|
|
Adult
|
T1T2
|
T3T4
|
N0
|
N1
|
N2N3
|
|
|
n =50
|
n = 27
|
n = 23
|
n = 8
|
n = 14
|
n = 28
|
|
PT eligibility
|
|
|
40.0 %
|
25.9 %
|
54.2 %
|
12.5 %
|
42.9 %
|
46.4 %
|
|
Passing rates
|
|
for threshold:
|
|
|
|
|
|
|
|
Single
|
36.0 %
|
22.2 %
|
50.0 %
|
12.5 %
|
42.9 %
|
39.3 %
|
|
Composite
|
20.0 %
|
11.1 %
|
29.2 %
|
12.5 %
|
21.4 %
|
21.4 %
|
Table 1:
Percentage of patients eligible for PT with MBS strategy and passing rates
relative to single and composite threshold. Values were shown relative to each
subgroup of the cohort stratification.
Conclusion
The
MBS strategy successfully drives the clinical identification of NPC patients,
who are most likely to benefit from IMPT. CTS well summarizes the expected
global gain.