Comparison of single and dual-energy CT based proton treatment planning for neuro patients
Tim van der Maas,
The Netherlands
PO-1504
Abstract
Comparison of single and dual-energy CT based proton treatment planning for neuro patients
Authors: Tim van der Maas1, Vicki Trier Taasti1, Ilaria Rinaldi1, Frank Verhaegen2, Gabriel Fonseca3, Wouter van Elmpt4
1Department of Radiation Oncology (Maastro), GROW School for Oncology, Maastricht University Medical Centre+, Maastricht, The Netherlands; 2Department of Radiation Oncology (Maastro), GROW School for Oncology, Maastricht University Medical Centre+ Maastricht, Maastricht, The Netherlands; 3Department of Radiation Oncology (Maastro), GROW School for Oncology, Maastricht University Medical Centre+ Maastricht Maastricht, Maastricht, The Netherlands; 4Department of Radiation Oncology (Maastro), GROW School for Oncology, Maastricht University Medical Centre+ Maastricht Maastricht Maastricht, Maastricht, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
To
fully exploit the benefits of proton therapy, an accurate stopping power ratio
(SPR) prediction is necessary. In
this study, we evaluated the dose differences between robustly optimized proton
plans based on single-energy CT (SECT) and dual-energy CT (DECT) for neuro-oncological
patients.
Material and Methods
57 patients treated with proton therapy
received a planning single energy CT (pSECT), followed by weekly repeat CTs acquired
in both SECT (reSECT) and DECT (reDECT) mode (SOMATOM Drive or Confidence,
Siemens Healthineers, Forchheim, Germany). On average, each patient had 4.6 sets
of paired reSECTs and reDECTs (range: 2-6). Clinical plans were created in RayStation 10A (RaySearch
Laboratories, Stockholm, Sweden). All plans were robustly optimized with a
range uncertainty of ±3% and an isotropic setup uncertainty
of 1 mm.
Commercial DirectSPR software (Siemens
Healthineers) was used to create SPR maps of the reDECTs. The contours on the reSECT
was copied to the corresponding reDECT. The clinical proton plan was robustly
re-evaluated on all weekly reSECTs and reDECTs. Dose-volume histogram (DVH)
parameters were extracted from the nominal dose distribution (nom) as well as
the voxel-wise minimum and maximum (VWmin/VWmax) dose distributions. As the
largest dose difference was expected distally to the target, only organs at
risk (OARs) overlapping the clinical target volume (CTV) expanded with 2 cm on
the pSECT were included in the evaluations. Moreover, ring structures of 1-5 mm
were created around the CTV and included in the analysis. The DVH-parameters for the CTVs, OARs and ring
structures were extracted from both the reSECTs and reDECTs, and the
DVH-differences were computed. For each patient, the averages of the
DVH-differences over the repeat CTs were computed.
Results
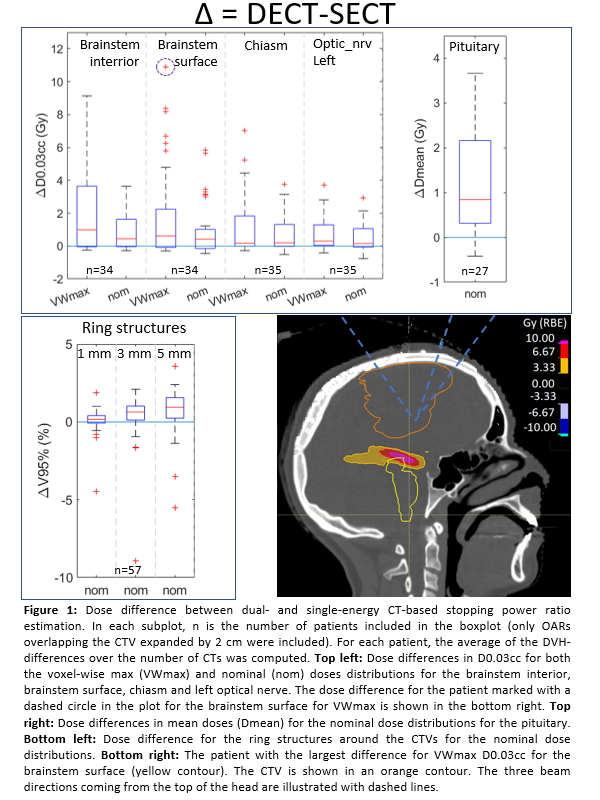
For
all patients, differences in CTV V94% for both the VWmin and nom dose
distributions were within -1.5% and 0.2%, both with medians of 0.0% (and Inter
Quartile Ranges (IQR) of 0.2% and 0.0%, respectively, Figure 1). The difference
in maximum dose (quantified by D0.03cc) to the CTV for both the VWmax as nom
were all within -0.7 and 0.6 Gy, with medians of 0.1 Gy (IQR=0.2 Gy) and 0.0 Gy (IQR=0.2 Gy), respectively.
For the OARs, larger differences were observed, of up to 10.9 Gy and 5.8 Gy,
with a median of 0.6 Gy and 0.4 Gy (IQR=2.3 Gy and 1.2 Gy) for D0.03cc in the VWmax
and nom dose distributions, respectively, for the brainstem surface (Figure 1
and 2). The proton ranges on the DECTs were generally larger
than on the SECTs causing dose differences (Figure 2). The same was seen for
the V95% for the ring structures in the nom dose distributions which showed an
increase in median difference up to 0.9% (IQR= 1.3%) with increasing ring size.
Conclusion
Dosimetric
differences in OARs were found between DECT and SECT-based dose distributions
due to different implementations of SPR calculation. These implementations have
negligible influence for CTV coverage but could influence OAR dose
calculations.