The clinical benefit of range uncertainty reduction in robust optimization for proton therapy
Ivanka Sojat Tarp,
Denmark
PO-1502
Abstract
The clinical benefit of range uncertainty reduction in robust optimization for proton therapy
Authors: Ivanka Sojat Tarp1, Vicki Trier Taasti2, Maria Fuglsang Jensen1, Anne Vestergaard1, Kenneth Jensen1
1Aarhus University Hospital, Danish Centre for Particle Therapy, Aarhus, Denmark; 2Department of Radiation Oncology (Maastro), GROW School for Oncology, Maastricht University Medical Centre+, Maastricht, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
For proton treatment planning, one of the main
causes of range uncertainty is on the CT-based estimation of stopping power
ratio (SPR) relative to water. SPR estimation is usually based on single energy
CT (SECT) scans, however, previous studies have shown that dual energy CT
(DECT) can result in a more accurate SPR estimation and thereby a reduction of range
uncertainty. In robust optimization, typically setup and range uncertainties
are accounted for, and lead to an expansion of the irradiated volume to assure
target coverage in all error scenarios. This study investigated how large a range
uncertainty reduction is needed to obtain a clinically relevant dose sparing to
organs at risk (OARs) for patients with brain tumours.
Material and Methods
This
study included treatment planning CT scan of ten patients with brain tumours acquired
in Twin Beam mode at a SOMATOM Definition Edge CT scanner (Siemens
Healthineers, Forcheim, Germany). The patients were treated with spot scanned
proton therapy and a prescription dose of 50.4Gy in 28 fractions. Based on the field
directions and optimization objectives of the original treatment plan, new
plans were robustly optimized with reduced range uncertainties using Eclipse
treatment planning system (Varian, Palo Alto, CA). Seven plans were created for
each patient, with a range uncertainty of 3.5% (original plan), 3.0%, 2.5%,
2.0%, 1.5%, 1.0% and 0.0%, respectively. For all robust optimizations, a setup
error of 2mm was used. Each plan was optimized until a clinically acceptable
plan (D95%>98% for clinical target volume (CTV)) was obtained for all
fourteen setup and range scenarios. The plans were normalized to CTV mean dose.
Dosimetric effect of a reduced range uncertainty was evaluated for OARs and ring
structures of various thickness (from 1mm to 20mm) surrounding the CTV. Comparisons
were made by evaluating mean and max dose to OARs and ring structures.
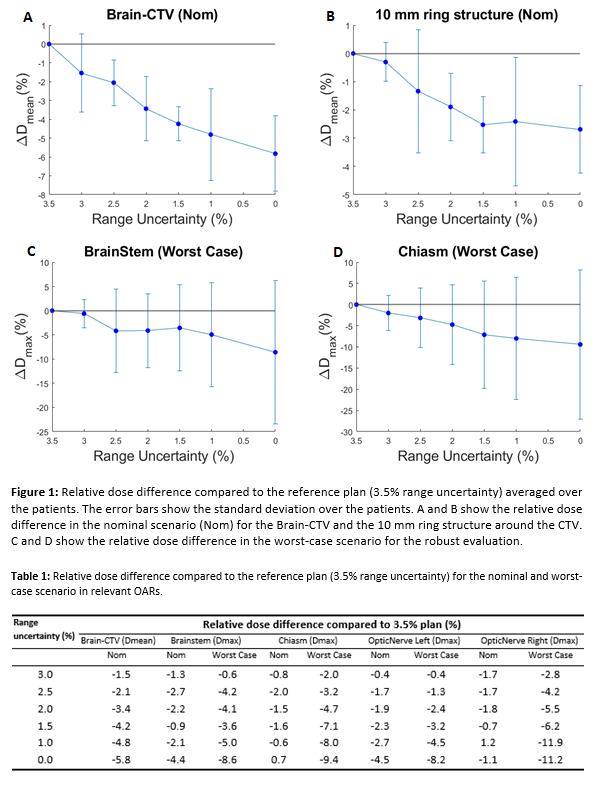
Results
The
results show that reducing the range uncertainty in the treatment plan gives a
slight reduction in nominal dose to the surrounding tissue. Mean dose to brain
outside CTV (Brain-CTV) is on average reduced by 3.4%(std=0.2%) over the ten
patients when reducing range uncertainty from 3.5% to 2%. Dose to the ring structure
of 10mm surrounding the CTV was reduced by 2% on average. In the worst-case
scenarios for Brainstem and Chiasm, reducing the range from 3.5% to 2.0%
results in a reduction in max dose by 4.1% (0.7Gy) and 4.7% (1.5Gy) respectively.
Table 1 contains the average dose differences for the evaluated OARs.
Conclusion
Reducing
the range uncertainty leads to meaningful reductions in dose to OARs. Whether
the reduction is clinically relevant or not needs to be seen in a context; if
the dose to OARs is close to the dose constraints maximum, the reductions shown
in this study are very relevant. To obtain a relevant dose reduction of 2% in
the mean dose to the brain outside CTV, new modalities such as DECT needs to
ensure that the range uncertainty can be reduced down to 2-2.5%.