Secondary cancer risk estimates from proton arc plans in pediatric craniopharyngioma.
PO-1785
Abstract
Secondary cancer risk estimates from proton arc plans in pediatric craniopharyngioma.
Authors: Laura Toussaint1, Daniel J Indelicato2, Jørgen BB Petersen3, Camilla H Stokkevåg4, Yasmin Lassen-Ramshad1, Anne Vestergaard1, Ludvig P Muren1
1Aarhus University Hospital, Danish Centre for Particle Therapy, Aarhus N, Denmark; 2University of Florida Health Proton Therapy Institute, Department of Radiation Oncology, Jacksonville, USA; 3Aarhus University Hospital, Department of Medical Physics, Aarhus N, Denmark; 4Haukeland University Hospital, Department of Oncology and Medical Physics, Bergen, Norway
Show Affiliations
Hide Affiliations
Purpose or Objective
In the past years, interest has been growing around the technological developments and dose tailoring benefits of proton arc therapy (PAT). One of the main concerns for the clinical application of PAT, especially when considering pediatric patients, is the potential enhanced risk of secondary cancer (SC) induction. Indeed, the low-dose bath is increased with PAT compared to state-of-the-art intensity modulated proton therapy (IMPT), and the impact of low doses on SC risks is still unclear. While a few studies investigated SC risk after passive-scattering PAT, reports on active-scanning PAT estimates are absent. The aim of this study was therefore to compare SC risks in pediatric craniopharyngioma patients if treated with either photon-based volumetric modulated arc therapy (VMAT), IMPT or active-scanning PAT.
Material and Methods
Treatment plans optimized in Eclipse with a prescribed dose of 54Gy(RBE) were generated on CT-scans from five pediatric craniopharyngioma patients using VMAT, IMPT and PAT-surrogate. The VMAT plans consisted of three arcs, the IMPT plans were calculated with three fields (right/left superior anterior oblique fields, and a superior posterior oblique field), and the PAT plans were arc surrogates plans, i.e. calculated with 18 equiangular beams with a minimal spot weighting of 0.01 monitor units (Fig. 1). The plans from the three treatment modalities offered equivalent target coverage. For the proton plans, relative biological effectiveness (RBE) variations were included by calculating both the proton dose with (1) a fixed RBE of 1.1 (as applied clinically), or (2) with variable RBE incorporating dose-averaged linear energy transfer (LETd), as RBE=1+0.055xLETd (PMB 2018;63:225009). The LETd distribution was calculated from the Eclipse treatment planning software.
Relative risks (RRs) of brain radiation-induced SC, based on the organ equivalent dose concept (Theor Biol Med Model 2011;8:27), were analyzed and compared by applying a brain-specific full mechanistic dose-response model for carcinoma induction.
Results
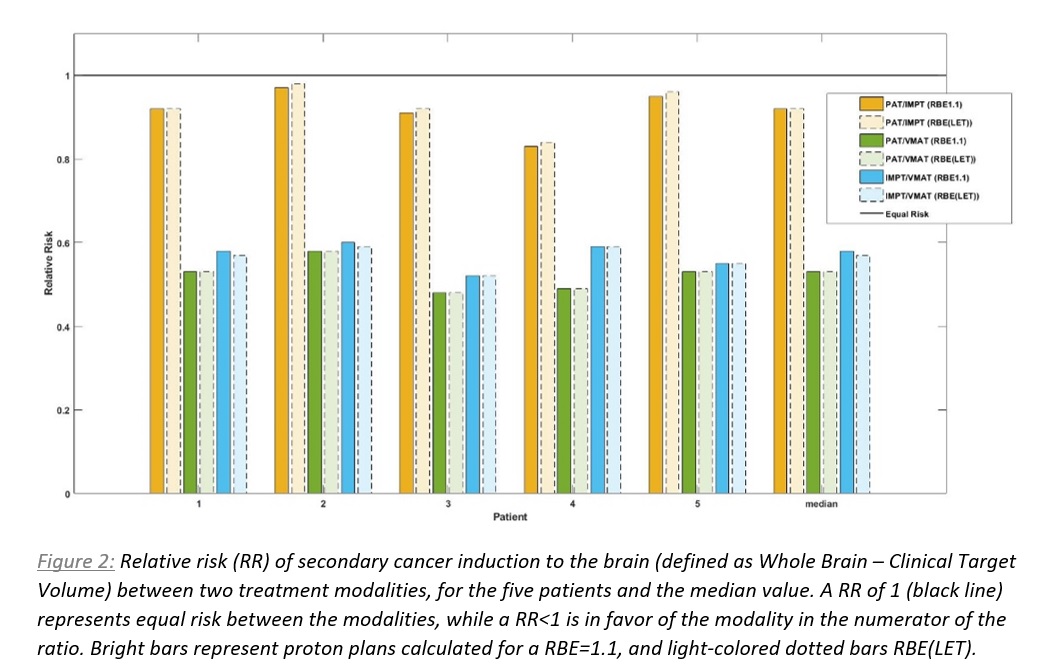
SC risks were lowest applying PAT-surrogate, with a median RR of 0.92 when compared to IMPT and 0.53 when compared to VMAT (RBE=1.1). The median RR for IMPT vs. VMAT was 0.58. For ranking of the plans according the SC risks, the same patterns were seen across all five patients (Fig. 2).
Including variable RBE did not influence the SC risks estimates considerably, especially when investigating PAT-surrogates where the median in RR was identical for RBE=1.1 and RBE(LET). For IMPT vs. VMAT, the median RR was 0.58 for RBE=1.1 vs. 0.57 for RBE(LET) (Fig. 2).
Conclusion
Despite an increased low-dose bath with PAT-surrogate vs. IMPT, a tendency towards SC risk-reduction was seen for PAT-surrogate in centrally located tumors. Compared to VMAT plans, SC risks were halved with both proton modalities. However, these results are based on the assumption that the RBE for cell inactivation and cell mutation/cancer induction are equivalent, which remains to be clarified.