Oxygen distribution in the microenvironment and RT treatment outcome: a modeling study on metastasis
PO-1774
Abstract
Oxygen distribution in the microenvironment and RT treatment outcome: a modeling study on metastasis
Authors: Luca Possenti1,2, Andrea Gallo2, Alessandro Cicchetti1, Tiziana Rancati1, Paolo Zunino3
1Fondazione IRCCS Istituto Nazionale dei Tumori, Prostate Cancer Program, Milan, Italy; 2Politecnico di Milano, LaBS, CMIC Dept., Milan, Italy; 3Politecnico di Milano, MOX, Department of Mathematics, Milan, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
Oxygen (O2) distribution is a known determinant of radiosensitivity, especially for low-LET ionizing radiation. It is delivered to the tissues (normal and tumoral) by the microvasculature and then depleted by cells. The work aims to evaluate different treatments (in terms of cell surviving fraction (SF) after RT) using a computational model of the microenvironment describing the O2 distribution and radiation damage. We analyze different RT sources and fractionations. We benchmark the model in clinical scenarios of lung metastasis grown in tissues with different vascularisation.
Material and Methods
To analyze two different metastasis scenarios, we investigated two possible vascular architectures: a high and low level of vascularisation (HV – network lateral surface 14.4 mm2/mm3tissue, LV – 3.3 mm2/mm3tissue). We computed the O2 distribution using the 3D1D computational model (Ann Biomed Eng 2021), which includes O2 consumption and accounts for the presence of red blood cells. We applied the linear-quadratic model following the Alper-Howard- Flanders formulation, which describes the SF dependence on O2 and LET. We tuned the parameters to reflect the radiosensitivity of lung metastasis (a = 0.08 Gy-1, a/b= 4 Gy). The model was complemented by the repopulation term, including a kick-off time of 14 days.
Three different fractionation schemes were analyzed (1x30 Gy[RBE], 3x10 Gy[RBE] – 1fr/w, 6x5 Gy[RBE] – 2fr/w) considering photons (LET = 2 keV/µm), protons (12 keV/µm, RBE = 1.1), and carbon ions (Cions, 100 keV/µm, RBE = 3).
Results
We obtained a poorly oxygenated scenario (LV – average pO2 14 mmHg) and an oxygen-rich one (HV - average pO2 48 mmHg). Our model describes the spatial distribution of tissue O2 (Figure 1a) and the 3D local map of the SF in the tumor tissue. We report the LV case irradiated with photons in the 3 x10Gy setting (Figure 1b). The heterogeneity of the SF is evident, with greater damage close to the vessels. After the 3 fractions and considering even repopulation occurring between the 2nd and the 3rd one, the difference in SF due to oxygen spans 3 orders of magnitude (9.5e-4 vs 0.19). Conversely, in the HV case, the same treatment scenario efficiently killed the tumor cells (max SF = 4.7e-5, Figure 1c).
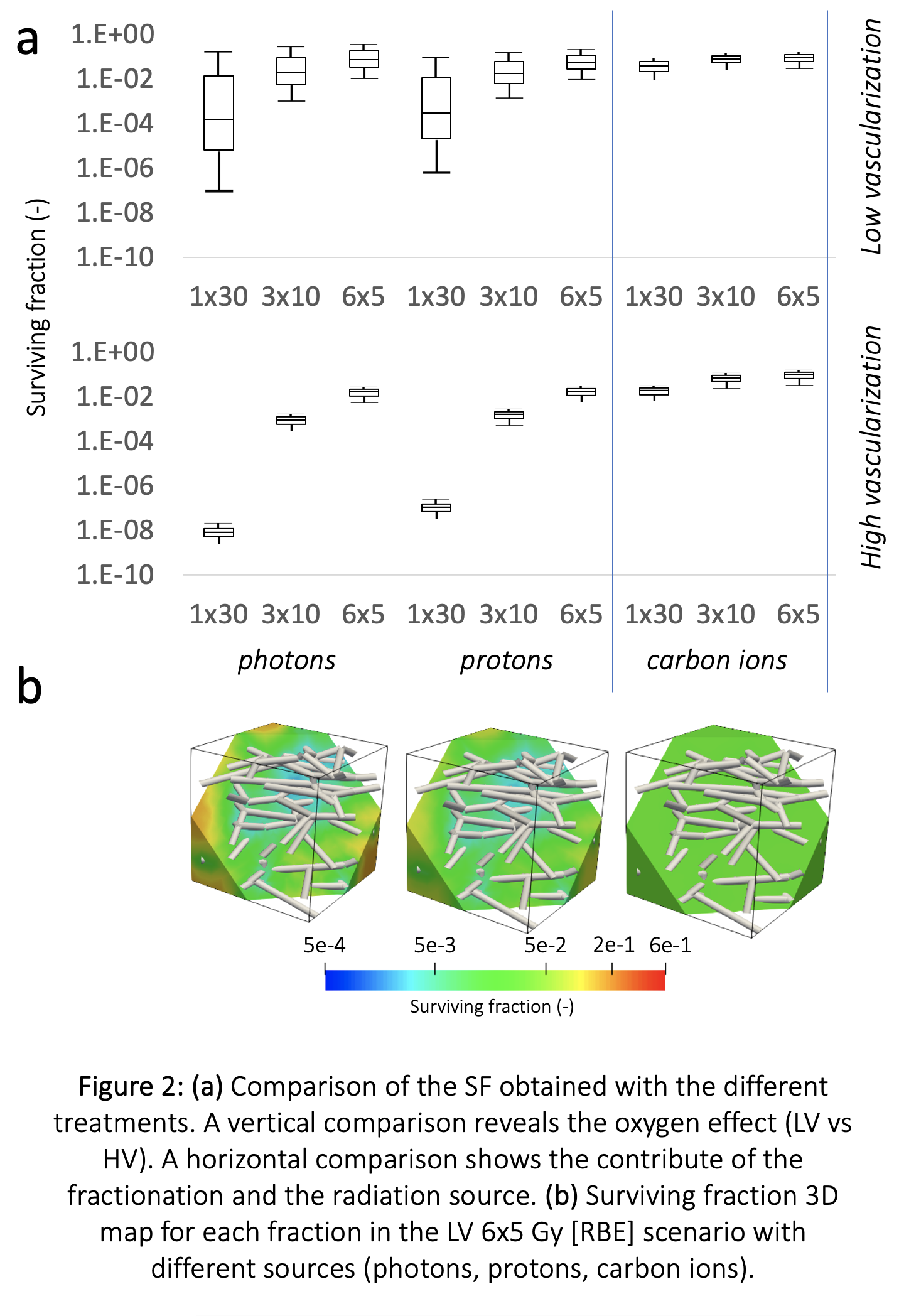
Comparing all the cases (Figure 2), the high dependence of photons irradiation on O2 is evident, determining different SF distributions. With more O2 available (HV cases) the SF distribution is less heterogeneous (6x5Gy photons – SF in LV: 1e-2 -> 2e-1, SF in HV: 5.2 -> 5.8e-3). The O2 effect is lower when greater LET is involved, such as in protons, but even more in Cions (6x5 Gy[RBE], SF in LV photons: 9.9e -> 3-1.9e-1, SF in LV protons: 9.3e -> 3-9.6e-2, SF in LV Cions: 2.8 -> 3.2e-2).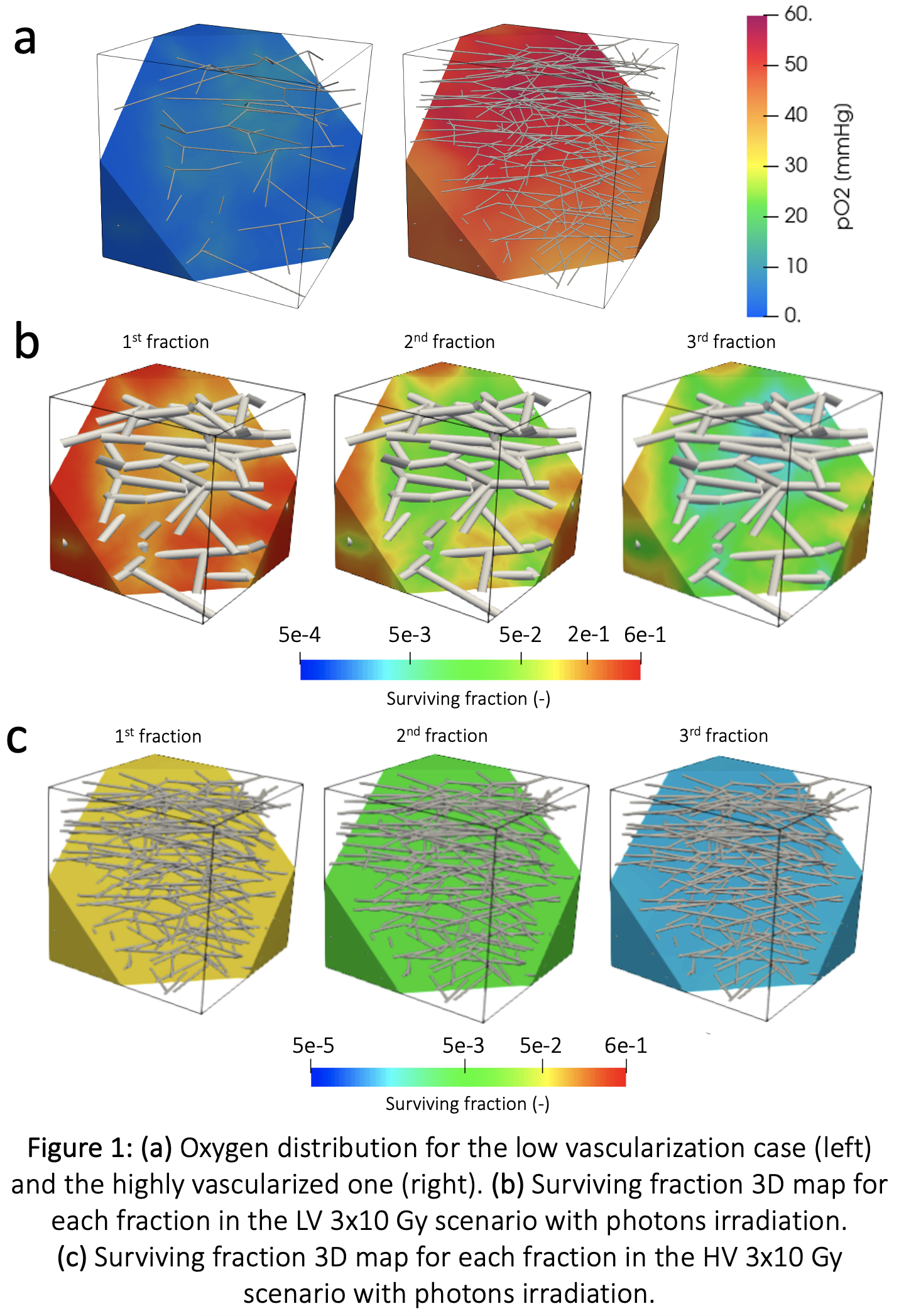
Conclusion
Applying our mechanistic model of oxygen delivery in the microenvironment, we analyzed the SF maps obtained with different RT treatments sources and schedules. Under poor oxygenation conditions, Cions therapy results in a more homogeneous SF map.
This work is supported by the AIRC Investigator Grant IG21479 Mechanistic computational modelling of radiation damage to microvasculature and of its effect on tumour microenvironment.