Dosimetric evaluation of esophagus segmentation for esophagus-sparing palliative spine irradiation.
Anna Mann Nielsen,
Denmark
PO-1443
Abstract
Dosimetric evaluation of esophagus segmentation for esophagus-sparing palliative spine irradiation.
Authors: Anna Mann Nielsen1, Claus P. Behrens1, Mette Riise Pedersen1, Lina Möller Andersson1, Morten Hiul Suppli2, Ivan Vogelius2,3, Patrik Sibolt1, Gitte Persson1,3
1Copenhagen University Hospital – Herlev and Gentofte, Dept. of Oncology, Copenhagen, Denmark; 2Copenhagen University Hospital – Rigshospitalet, Dept. of Oncology, Copenhagen, Denmark; 3Copenhagen University , Dept. of Clinical Medicine, Copenhagen, Denmark
Show Affiliations
Hide Affiliations
Purpose or Objective
We have
initiated the ESO-SPARE phase III trial where patients with metastatic spinal
cord compression are randomized to either esophagus sparing- or standard IMRT
with patient reported esophagus toxicity as primary endpoint. Another aim is to
reduce time to treatment by using artificial intelligence (AI) software to automate
the planning process.
First step
is to use AI automatic segmentation of the esophagus. Manual delineations are
prone to interobserver variation and we hypothesize that the variation between automatic
segmentation and manual delineation has the same magnitude as interobserver
variation. Furthermore, we hypothesize that the dosimetric consequence of using
the automatic segmented esophagus for plan optimization is minimal compared to
a manual delineated esophagus.
Material and Methods
The
planning CTs of ten consecutive patients referred for palliative radiotherapy
for metastatic spinal cord compression (MSCC) in the thoracic spine were included
in this analysis. The scans were acquired with a 2-mm slice thickness on a
Siemens SOMATOM go.Open Pro with syngo CT VA30A software installed (Siemens
Healthineers ™). Automatic segmentation of the esophagus (eso-AI) was done using
the scanner software. Two radiation oncologists retrospectively delineated the
esophagus in full length (eso1/eso2) - blinded to the eso-AI and each other.
The esophagus’s lengths were adjusted to start and stop in the same slices. VMAT
plans with two 360 degree-arcs were created for each of the ten patients with
optimization on each of the three eso-volumes. Prescribed dose was 25 Gy/5
fractions. The esophagus constraint (D(0.027cc) < 8.5 Gy) was
prioritized higher than the PTV constraint (V90% > 95%). Delineation
and treatment planning were performed in eclipse (Varian medical systems ™).
DICE similarity scores were calculated for eso-AI/eso1, eso-AI/eso2 and
eso1/eso2. Dose coverage was compared for the three plans optimized on each
patient. Friedmans test are used for statistical analysis and results are
reported as median (range).
Results
The mean
DICE similarity scores were significantly smaller between eso-AI and the two
observers (0.69-0.84 and 0.70-0.83) than between observers (0.79-0.88) (p<.0005).
The eso-AI had a significantly smaller volume than both eso-1 and -2 (p=0.02).
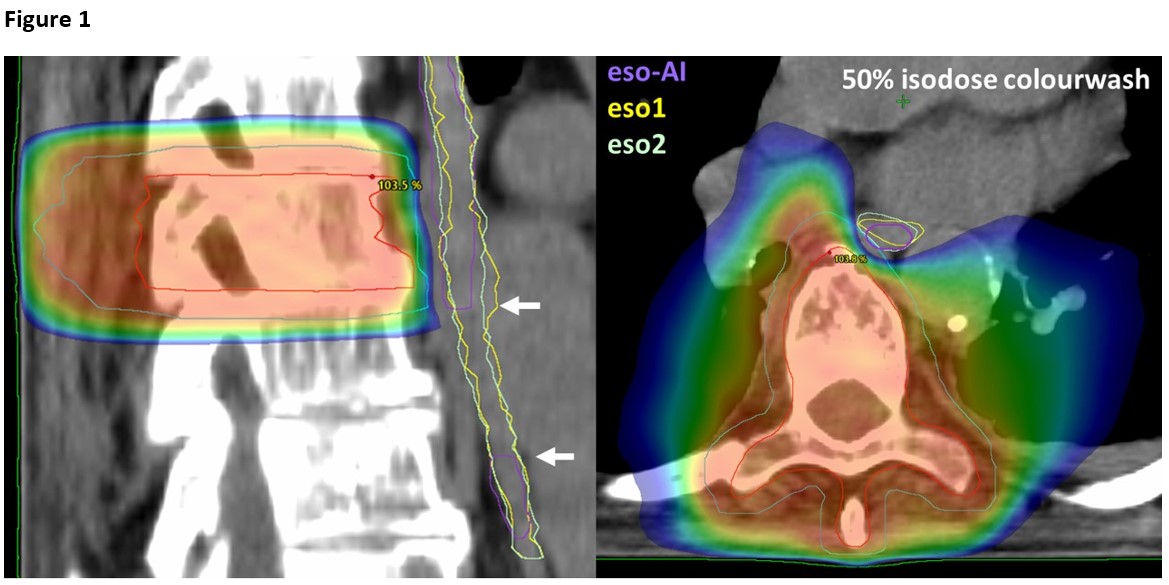
In one case, the eso-AI was not segmented in all relevant slices, Figure 1. There
was no difference between target coverage, measured as PTV V90%, GTV
V95% and GTV Dmean, in plans optimized on the different
esophagus volumes. When the eso-AI was used for optimization it led to
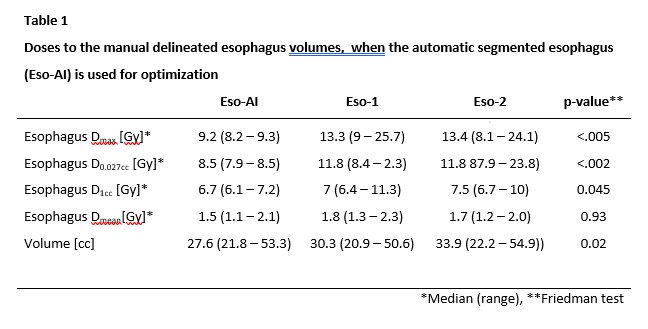
overdosing of eso1 and eso2, Table 1.
Conclusion
The
automatic segmented esophagus was smaller than the manual delineated esophagi and
even failed to segment the entire esophagus in a single case. When used for optimization it led to violation
of the dose constraint for the manual delineated esophagi. However, the
clinical impact is unknown. The automatic segmented esophagus can not be
recommended for clinical use without manual correction.