First results of the Leiden-Holland Proton Therapy Center collaboration for uveal melanoma treatment
PO-1429
Abstract
First results of the Leiden-Holland Proton Therapy Center collaboration for uveal melanoma treatment
Authors: Nanda Horeweg1, Elise Verbeek2, Khanh Vu2, Marina Marinkovic2, Jaco Bleeker2, Myra Rodrigues3,1, Yvonne Klaver3,1, Jan-Willem Beenakker2,1,4, Gré Luyten2, Coen Rasch1
1Leiden University Medical Center, Radiation Oncology, Leiden, The Netherlands; 2Leiden University Medical Center, Ophthalmology, Leiden, The Netherlands; 3Holland Particle Therapy Center, Radiation Oncology, Delft, The Netherlands; 4Leiden University Medical Center, Radiology, Leiden, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
To evaluate clinical outcomes, including local tumour control, visual
acuity, adverse events and eye preservation, of uveal melanoma patients treated
at proton therapy centre HollandPTC in the Netherlands.
Material and Methods
Since December 2019, patients diagnosed
with uveal melanomas at Leiden University Medical Center (LUMC) are offered proton
therapy at HollandPTC if the tumour height (including sclera) is >7-8mm and/or the tumour
diameter is >16mm and/or the tumour is located juxtapapillary.
Patients with smaller tumours are offered Ruthenium-106 brachytherapy. Enucleation
is an alternative for those not willing or able to undergo radiotherapy. For
this cohort study, we included all consecutive patients referred from LUMC for proton therapy treated up to January
31st 2021. All patients were treated with 60.0 GyE in 4 fractions
using Eclipse Ocular Proton Planning system (Varian Medical Systems, Inc.). Before treatment, tantalum localisation clips were placed on the sclera. Toxicities
after proton therapy were classified according to CTCAE v4.0. Time-to-event
analyses and actuarial survival rates were determined using Kaplan Meier
method.
Results
A total of 28 patients were included for analysis with a mean age of 61
years. The median initial tumour diameter and thickness were 15.2 mm (range:
7.0-20.5) and 8.3 mm (range 2.5-14.8). 39% of the tumours were located
centrally, 43% mid-peripherally and 18% peripherally. At baseline 50% of the
patients had a visual acuity <0.5 and 25% <0.1. The median follow-up time
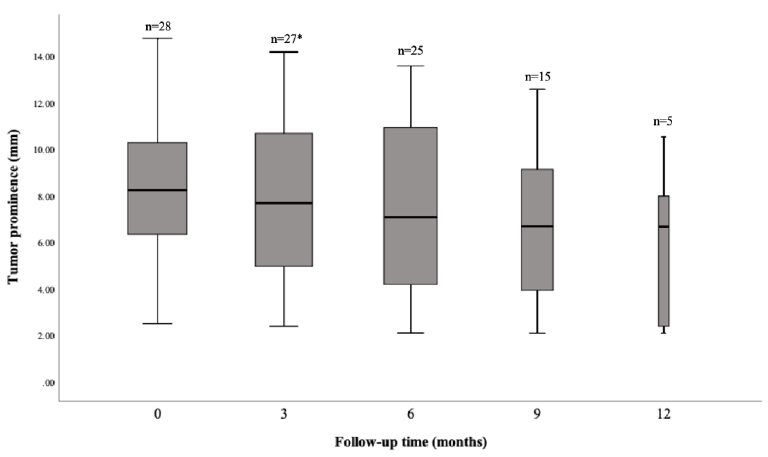
was 9.5 months. Tumour thickness regressed slowly after treatment and is
presented in Figure 1. At one year after treatment, local tumour control,
metastasis-free survival and overall survival were respectively 100%, 96% and
89%; the eye preservation rate was 96%. At one year, visual acuity was <0.5
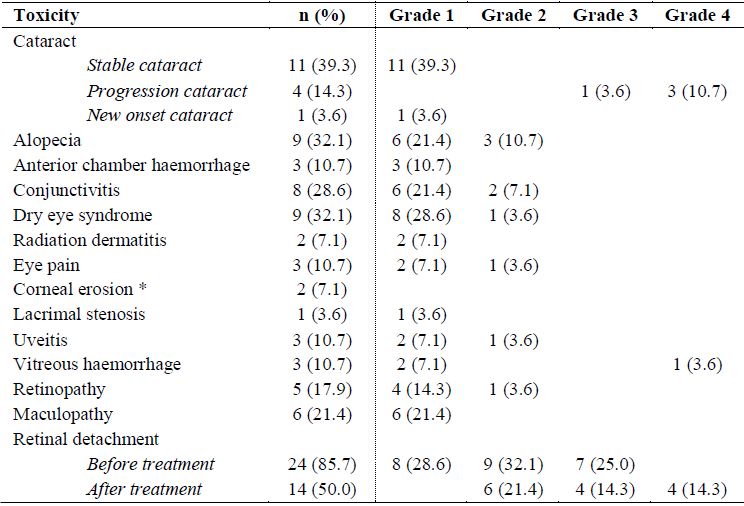
in 89% and <0.1 in 62%. Ocular toxicities were common during the first year.
The majority of toxicities were grade 1-2, but some invasive procedures had to
be performed for mature cataract, vitreous haemorrhage and retinal detachments
(Table 1).
Conclusion
Proton therapy is now available for uveal melanoma patients in the
Netherlands at HollandPTC as alternative for enucleation or referral for proton
therapy in Switzerland. As such, the patient selection has changed and now
includes more patients with central tumours and low initial visual acuity. This
is reflected in the visual outcomes and risk of toxicity. Nonetheless, local
tumour control and survival outcomes were excellent.