Brachytherapy plan reproducibility in gynecological cancer patients
PO-1488
Abstract
Brachytherapy plan reproducibility in gynecological cancer patients
Authors: Elisa Placidi1, Laura Breschi2, Guenda Meffe2, Valentina Lancellotta2, Francesca Greco2, Calogero Casà2, Gerardina Stimato2, Bruno Fionda2, Stefania Teodoli2, Maria Antonietta Gambacorta2, Luca Tagliaferri2, Luca Indovina2
1Fondazione Policlinico Universitario Agostino Gemelli IRCSS, Radiation oncology, Rome, Italy; 2Fondazione Policlinico Universitario Agostino Gemelli IRCSS, Radiation Oncology, Rome, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
The treatment planning of radiotherapy treatments is a key step for the delivery of the prescribed dose to the target while sparing the organs at risk (OARs) as much as possible. In brachytherapy, or interventional radiotherapy (IRT), the optimization of the plan is strictly dependent on the position of the applicators, therefore for gynecological treatments with intrauterine devices, the plan is performed for each fraction, leading to a very time-consuming procedure. This study aims at the evaluation of the dosimetric differences of the plans of the first and the second day of treatment in order to investigate the possibility of avoiding the execution of the plan on the second day.
Material and Methods
6 patients with cervical cancer, treated with intracavitary high dose rate IRT with a Fletcher applicator (®Elekta), were enrolled in this study. On the day of the treatment, the patients were prepared for the by using a rectal probe and a Foley catheter. The protocol is based on GEC-ESTRO recommendation. The first IRT fraction is MR/CT based, the 2nd, 3rd and 4th fraction is CT based. For each treatment, a planning CT was acquired, and the treatment plan was performed with Oncentra 4.6 TPS (®Elekta) using the TG 43 algorithm. The CTV and the OARs were manually contoured, and the applicators were manually reconstructed. The dosimetric parameters used for the evaluation of the plan were the V100, the V95 and the V90 for the CTV, whilst for the OARs (bladder, bowel and rectum) the D2cc was considered. At the end of the first and third fraction the applicator was not removed and the following day the same procedure was performed (2nd and 4th fraction). A virtual second treatment plan was performed on the CT images of the second day by copying the same dwell positions and times from the first fraction. The dosimetric differences were evaluated as percentage variation in volume.
Results
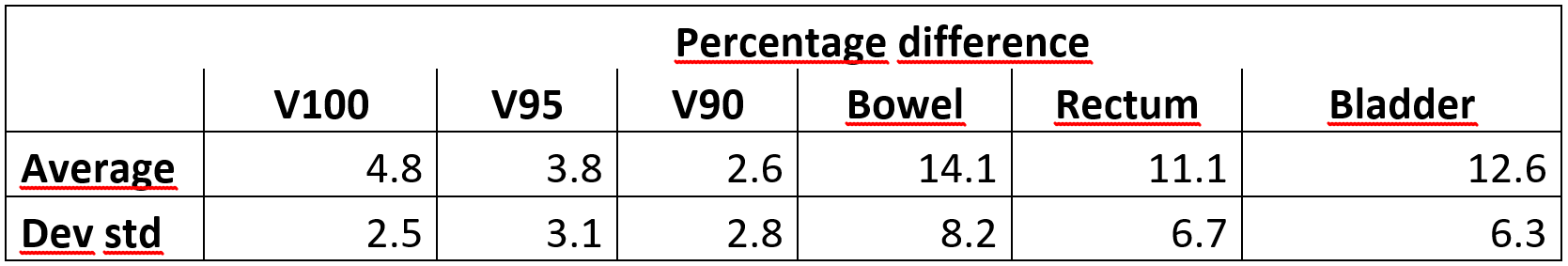
The table reports the percentage variations of the dosimetric parameters
considered for the CTV (V100, V95 and V90) and the D2cc of the OARs. The
constraints for the CTV resulted below 5%, meaning that the plan performed for
the first fraction would be clinically acceptable for the CTV also for the second
fraction. On the other hand, the percentage difference of the OARs are well
above 5% meaning that even if the implant is the same, the plan optimized for
the first day of treatment would not be clinically acceptable for the second
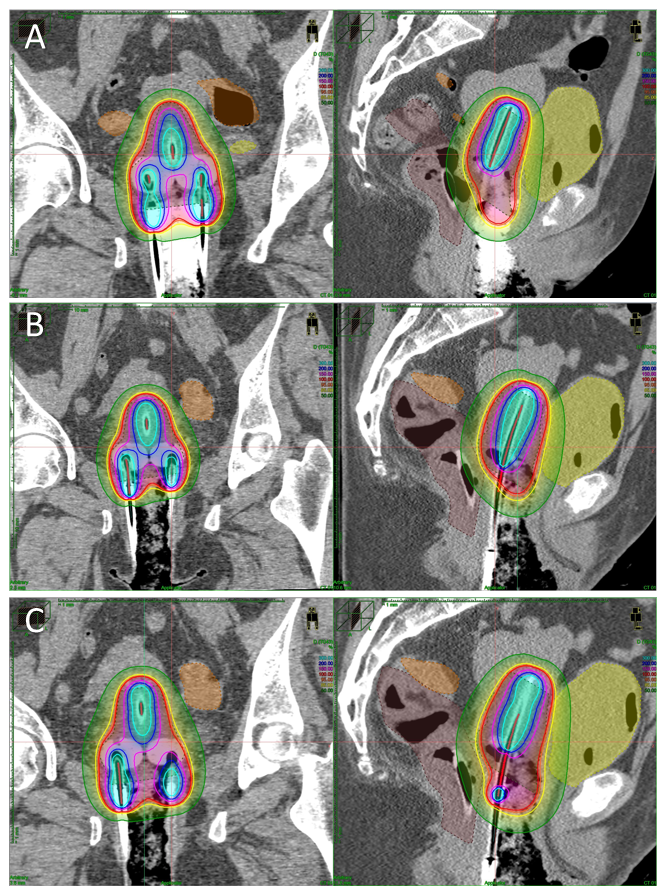
day. Figure 1 shows the dose
distribution in the first fraction (A), the original manually optimized second
fraction (B) and the second fraction copied from the first one (C).
Conclusion
This study showed a dosimetric comparison
between identical plans performed in two consecutive days of treatment. A
percentage variation of more that 5% was found for the OARs suggesting that the
interfraction variability needs to be further investigated and addressed.
Future work will therefore include a greater number of patients to be enrolled
and different applicators to be investigated.