Contour guided deformable image registration for adaptive radiotherapy
PO-1485
Abstract
Contour guided deformable image registration for adaptive radiotherapy
Authors: Lando Bosma1, Mario Ries2, Baudouin Denis de Senneville3, Bas Raaymakers1, Cornel Zachiu1
1UMC Utrecht, Department of Radiotherapy, Utrecht, The Netherlands; 2UMC Utrecht, Imaging Division, Utrecht, The Netherlands; 3CNRS/University of Bordeaux, Institut de Mathematiques de Bordeaux (IMB), Talence, France
Show Affiliations
Hide Affiliations
Purpose or Objective
Deformable image registration (DIR) is a core element in the development of
adaptive radiotherapy workflows, integrating daily contour propagation and/or
dose accumulation within their design. Prior to the daily therapy session
however, the contours generated by DIR may undergo manual adjustments by the
operator, which in turn locally invalidate the estimated deformation vector
field (DVF). With the new contours and accumulated dose no longer in
correspondence, the DVF requires a re-calculation in accordance with the
corrections of the operator.
Here, we present a novel DIR algorithm that incorporates manual contour
information to guide automatic registration results.
Material and Methods
We propose estimating the adjusted deformations as the
minimizer of the following cost function:
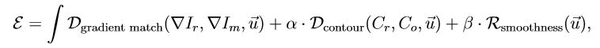
where the first term aims to
align similar contrast patterns in the reference and moving images (as proposed
in [1]). The second term is the sum of squared differences between the
reference and the daily “operator-validated” contours. The final term constrains the
estimated deformations to be spatially smooth, making the problem mathematically
well-posed.
We have validated the
algorithm on 20 clinical “planning MR” to “daily MR” registrations for prostate cancer patients,
with clinical contours available for both image sets, as well as on a dataset
generated using biomechanically-simulated deformations. The algorithm is
compared against the EVolution algorithm [1], which employs the same cost
function as above but without the second contour-guidance term. The algorithm is evaluated in
terms of the Dice similarity coefficient, and the endpoint error and dose error
for the biomechanical simulation.
Results
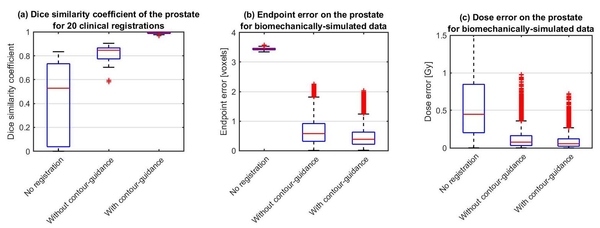
As expected, our contour-guided algorithm increases the mean Dice
similarity coefficient for the 20 prostate registrations from 0.81 for EVolution
without contour-guidance to 0.99, see Figure 1a. More importantly, compared to EVolution
without contour-guidance, the mean endpoint error on the prostate decreases by a factor of 1.4 from 0.65 mm to 0.46 mm for the simulated prostate case (see Figure 1b) and the mean
absolute dose error on the prostate decreases by a factor of 1.4 from 0.13 Gy to 0.09 Gy, see
Figure 1c. Also, no new errors are created by using our proposed method.
Conclusion
We introduce a contour-guided DIR algorithm that adapts and improves the
registration results for applications involving dosimetric information. This
provides a solution for when a registration result is unsatisfactory and makes
sure the DVF and warped dosimetric information are in accordance with the
operator-validated warped contours. This thus presents a feasible
semi-automatic strategy for spatially correct dosimetric information even in
difficult and artefacted cases.