Evaluation of Hyaluronic acid dissolution with hyaluronidase in an in-vitro prostate cancer model
PO-1418
Abstract
Evaluation of Hyaluronic acid dissolution with hyaluronidase in an in-vitro prostate cancer model
Authors: Ben Vanneste1, Ludy Lutgens2, Evert Van Limbergen2
1MAASTRO, Department of Radiation Oncology, Maastricht, The Netherlands; 2MAASTRO, Radiation Oncology , Maastricht, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Hyaluronic acid
(HA) is one of the four commercialized implantable rectum spacers available on
the market, besides hydrogel, saline filled balloon, and human collagen. These
implantable rectum spacers are used to decrease rectal radiation dose in
prostate cancer radiotherapy to avoid rectal complications after prostate
cancer radiotherapy. However implantation of such devices are not risk-free: if
such spacers are accidentally implanted in the rectal wall, might result in a
chronic wound. Then it can take 3 to 9 months for such spacers to resolve.
Furthermore, such a complication results in a significant treatment delay,
since an immediate start of radiotherapy could increase the risk of fistula
development. So, the feasibility to resolve such a spacer is of great interest
in the community. To determine a dose response relationship of disintegration
between a hyaluronic acid (HA) used in prostate cancer radiotherapy and
hyaluronidase (HAS).
Material and Methods
Five in-vitro prostate
cancer models (SIMTM) are applicated with 3 milliliter (ml) HA (Figure 1For
dissolution varying doses of HAS were used: 6 ml, 3 ml, 1.5 ml, and 0 ml. One
ml contains 150 International Units (IU). Each HAS was added with saline till
the complementary amount of 6 ml. One phantom was solely implanted with a HA 3 ml
acting as a control. Length, width and height were measured on different time
points: 1st day 4 times, 2nd day 3 times, third day 2
times, and then once daily during one week, with a final measurement 2 weeks
after implantation.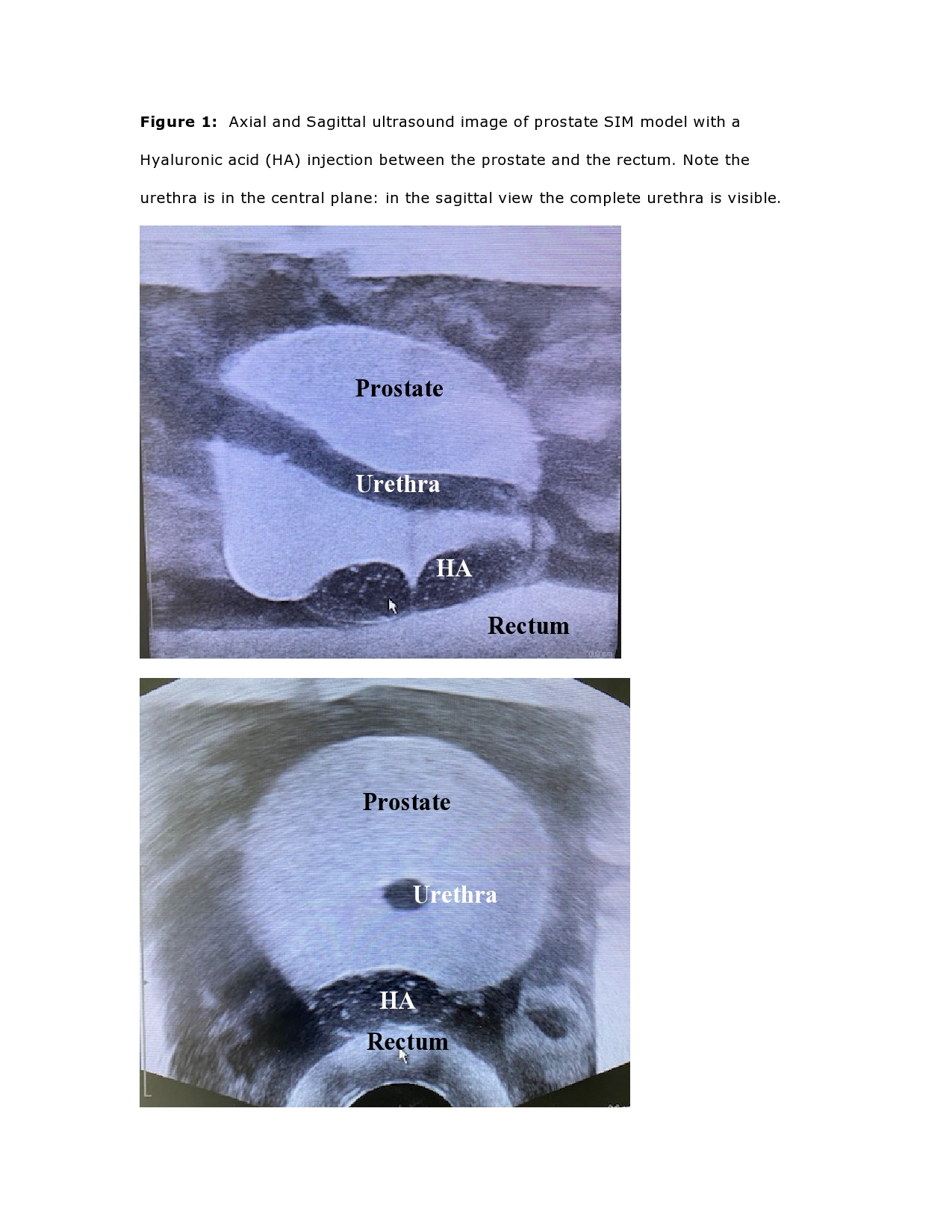
Results
The fastest
dissolution was observed with the highest concentration of HAS, starting
already at the first time point 2 hours after implantation, with volume
decrease of 50% on the second day, and less than 1 ml residue (33%) on day 4.
The 2 other concentrations of HAS also showed a volume decrease, with less than
2 ml (66%) on day 4. All the applied quantities of HAS are observed with a
residue of less than 1 ml after 7 days. After 14 days the control phantom and
the saline filled one remains on steady state volume (3 ml).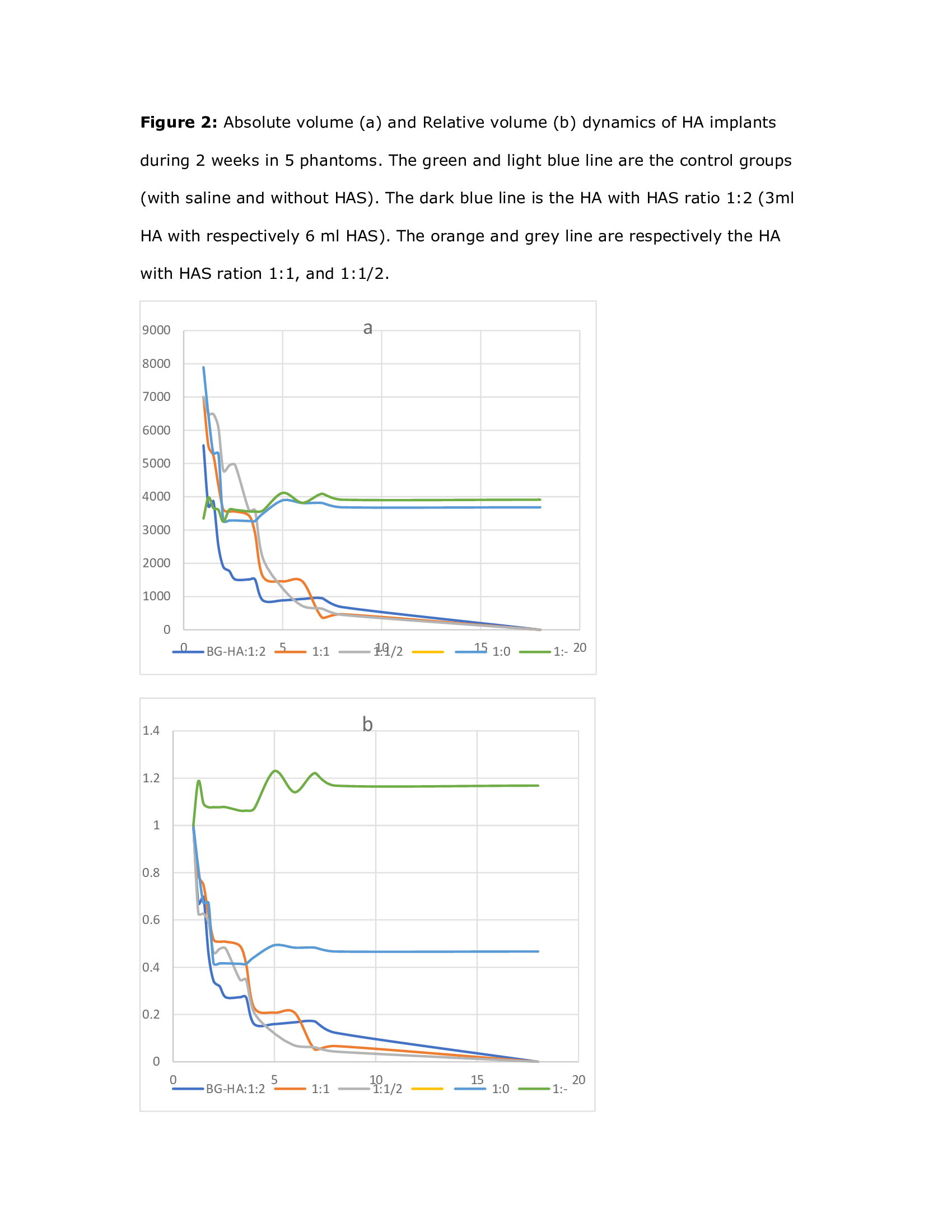
Conclusion
A dose response
was observed by HAS injection: highest volumes of HAS dissolute most swiftly. A
ratio of HA:HAS of 1:2 has already a decrease of half of volume on the second
day. This is of special interest while using the HA in clinical practice when
wrongly positioned, and dissolution is urgently needed.