Sbrt in prostate cancer: a feasibility and safety annalysis
Jose Antonio Solis Campos,
Chile
PO-1405
Abstract
Sbrt in prostate cancer: a feasibility and safety annalysis
Authors: Jose Antonio Solis Campos1,2, Jorge Olivares Gonzalez1, Hernan Letelier Flores3, Gabriel Lazcano Alvarez1, Ilan Perrot Rosenberg1, Gabriel Veillon Contreras1,2, Benjamin Tudela Staub1,2, Ruben Yañez Davila3, Cristian Herrera Bovet3, Yaniro Guillen Cabrera3, Gonzalo Rubio Riquelme4
1Universidad de Valparaiso, Oncology, Valparaiso, Chile; 2Hospital Carlos Van Buren, Oncology department, Valparaiso, Chile; 3Hospital Clínico Regional de Valdivia, Oncology, Valdivia, Chile; 4Universidad Diego Portales, Oncology, Santiago, Chile
Show Affiliations
Hide Affiliations
Purpose or Objective
Radiotherapy is a therapeutic option in localized prostate cancer and ultra-hypofractionated regimes (SBRT) are a validated alternative in terms of effectiveness and safety. The purpose of this study is to describe the treatment characteristic and acute toxicity in a multicentric prostate cancer patient cohort treated with SBRT
Material and Methods
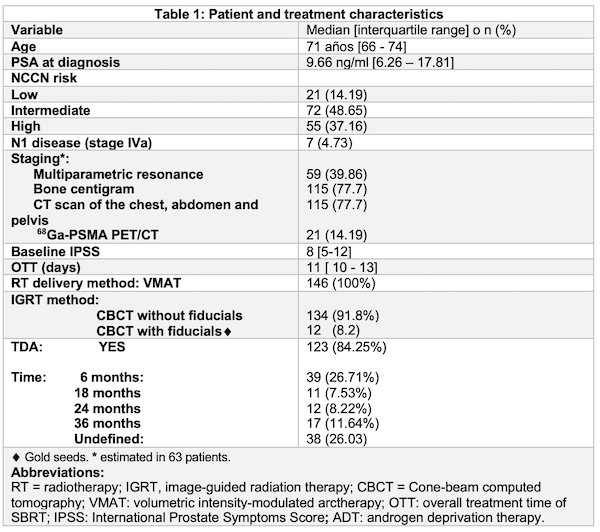
We performed a retrospective analysis of patients treated with SBRT with a curative intention between 2020-2021 at Carlos Van Buren Hospital - Valparaiso and Valdivia Regional Hospital with a flattening filter free volumetric modulated arc therapy. Online correction was performed before each session. Acute toxicity was defined according to RTOG criteria. In both centers, CTV was defined as: 1) Prostate (low risk patients) 2) Prostate + 1 cm Seminal Vesicles (S.V) (intermediate risk) 3) Prostate + 2 cm S.V (high risk) PTV = CTV +0.5 cm (0.3 cm posteriorly). At Van Buren Hospital Prescription consisted of 36.25 Gy in 5 fractions (alternate days) to Prostate PTV and 27.25 Gy in 5 fractions to S.V PTV. Valdivia’s prescription consisted of 36.25 Gy in 5 fractions uniformly to PTV escalating up to 40 Gy with a simultaneous integrated boost technique to prostate in intermediate and high-risk patients. This study was approved by the local ethics committee.
Results
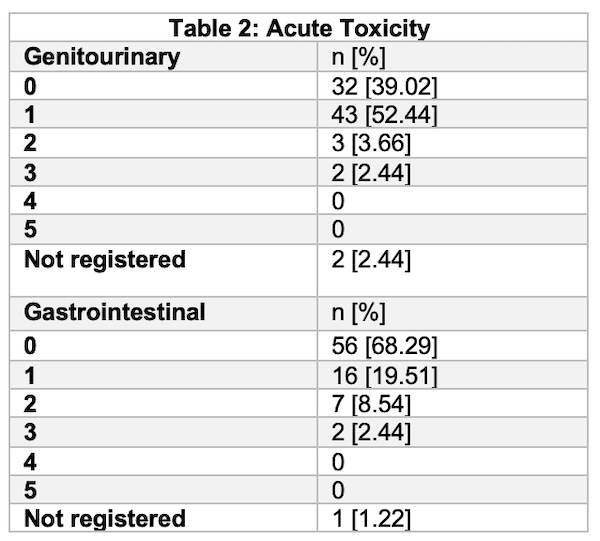
146 patients were included. 55 (37.16%) patients had high risk disease. 118 (79.7%) patients had staging procedures. In 87 (58.8%) patients planning was based on MRI / CT fusion. Gold seeds (fiducial markers) were used In 12 patients. Median PSA was 9.66 (IQR 6.26-17.8). 99 (66.9%) of patients had some sort of acute toxicity. 2 patients suffered from acute G3 gastrointestinal toxicity and 2 patients from acute G3 genitourinary toxicity. No G4-G5 events were observed. One patient had to suspend treatment due to urinary obstruction caused by disease progression.
Conclusion
SBRT is a safe therapeutic approach in prostate cancer patients with an excellent tolerance and acute toxicity profile. Efficacy results will be reported once appropriate follow up is reached.