Dose-adaptive MR-linac workflow for hypofractionated focal boost radiotherapy in prostate cancer
Linda G.W. Kerkmeijer,
The Netherlands
PO-1371
Abstract
Dose-adaptive MR-linac workflow for hypofractionated focal boost radiotherapy in prostate cancer
Authors: LGW Kerkmeijer1, Martina Kunze-Busch2, Robert Jan Smeenk1, Peter Van Kollenburg1, Leontien Abbenhuis1, Tamara Scheenstra1, Luca Engels1, Chen-Xi Van der Sande1, Narda Verheyden-Beckers1, Loes Spee1, Levi Hinke1, Ellen Brunenberg1, Erik Van der Bijl1
1Radboud UMC, Radiation Oncology, Nijmegen, The Netherlands; 2Radboud UMC, Radiation Oncology , Nijmegen, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
The
addition of a focal boost to the intraprostatic lesion(s) improves outcome for
patients with intermediate and high risk prostate cancer. Furthermore, this can
be achieved with no additional toxicity or impact on quality of life, as was
demonstrated in the multicenter phase III randomized FLAME trial. The purpose
of the present analysis was to evaluate the technical and clinical feasibility
of a novel dose-adaptive workflow on an 1.5T MR-linac for an
ultra-hypofractionated focal boost scheme.
Material and Methods
Until
October 2021, 20 patients with intermediate and high risk localized prostate
cancer were treated at the Radboudumc on the MR-linac in 5 fractions of 7 Gy
with an iso-toxic focal boost up to 50Gy within a multicenter phase II study.
The focal boost dose was reduced if necessary to adhere to the constraints of
the organs at risk. In a fully adaptive Adapt-To-Shape (ATS) workflow the
contours of the reference MRI were propagated to the daily MRI and manually
adapted when required within a ring of 1cm around the target volume. Based on these
contours the reference plan was adapted using a full re-optimisation. The
optimisation goals (iso-constraints) of the GTV, CTV and OARs for the daily online plan were
manually adjusted to acquire an optimal balance of a GTV dose as high as
possible, within the OAR constraints. A maximum of three optimisation rounds with
iso-constraints adaptation was allowed to prevent prolonged intrafraction time.
After completion of plan optimisation, the ATS workflow was followed by a
position verification scan. When intrafraction motion was ≥2 mm, an additional
translation (Adapt-To-Position) was performed. During beam-on, a 3D T2 weighted
image was acquired. After each fraction, all contours were adapted by an
experienced RTT on the beam-on scan and the delivered dose per fraction for all
structures was accumulated. The actual delivered doses of all previous fractions
were taken into account for adjustment of the iso-constraints in the remaining
fractions when deemed necessary or the creation of a new reference plan, see
Figure 1 for a visual of the workflow.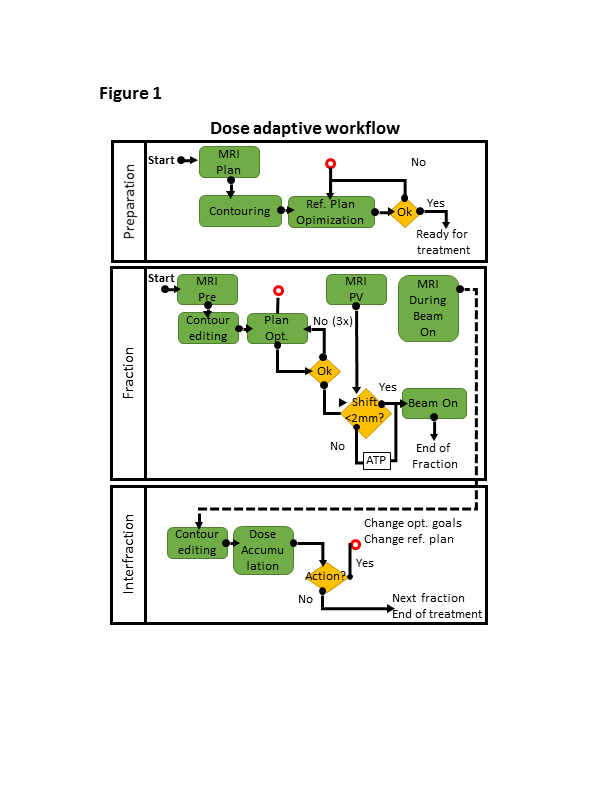
Results
All
fractions were delivered at the MR-linac and it was feasible to
obtain the actual delivered dose of the previous fractions before the
subsequent fractions and take this into account in the online plan
optimization process. Based on the accumulated dose, in 14 out of the 20 patients
the online optimization goals were manually adjusted. In 3 out of the 20 patients
a new reference plan was created for the remaining fractions. This was
performed when the OAR constraints during beam-on were largely exceeded due to
intrafraction motion.
Conclusion
A novel dose-adaptive
workflow based on the actual delivered dose of the target volume and OARs in
previous fractions is feasible for ultra-hypofractionated radiotherapy with an
iso-toxic focal boost. Future studies should focus on automatization and
artificial intelligence solutions to allow for rapid online dose-adaptive
workflows.