Prognostic role of ALBI-T in locoregional advanced hepatocellular carcinoma (BCLC-C) undergoing SBRT
PO-1295
Abstract
Prognostic role of ALBI-T in locoregional advanced hepatocellular carcinoma (BCLC-C) undergoing SBRT
Authors: Deepti Sharma1, Deepak Thapar1, Rose Kamal1, Hanuman Prasad Yadav1
1Institute of Liver and Biliary Sciences, Radiation Oncology, New Delhi, India
Show Affiliations
Hide Affiliations
Purpose or Objective
The Barcelona Clinic Liver Cancer stage
C(BCLC-C) is defined by the presence of PVTT and/or extrahepatic spread. Such patients are often
treated with systemic therapy as there is no consensus on use of loco regional
therapy in advanced/ metastatic HCC. Palliative SBRT is delivered with an aim
to improve overall survival (OS), quality of life and local control of disease.
However, the extent of tumor burden
and impairment in liver function may affect patient prognosis as well as
treatment outcome. ALBI-T is one of the scoring system which predicts survival
in advanced HCC and incorporates both tumor burden and LFT (S. Albumin and S.
Bilirubin)
The Aim of the study is to sub-classify
patients of BCLC-C on basis of tumor burden and LFT by using ALBI-T scoring
system and further evaluating the efficacy of SBRT.
Material and Methods
It
is an observational retrospective study done between May 2020 and Sep 2021. The
data was collected from 33 patients with BCLC-C HCC, who were unsuitable for
other liver-directed therapies. Patients with Child pugh score A5-B7 along with
normal liver reserve ≥ 700cc were included. All patients were classified before
SBRT by the ALBI-T scoring system. Cumulative survival rates were calculated
using the Kaplan-Meier method and were compared using the log-rank test.
Results
In the cohort of 33 patients (table 1),
Portal vein and IVC tumor thrombosis were present in 97% and 21% patients
respectively. Lung and nodal metastasis were found in 33% and 79% patients
respectively.
Median tumour volume was 600cc (100 -
1925). The median SBRT dose prescription was 35Gy (25-45Gy) in 5 to 10
fractions.
The 1 year LC and PFS were 90% and 40%
respectively. The most common site of progression in patients were as follows:
lungs (11) followed by nodes (6), liver (4) and bone (2).
The median survival of patients in entire
cohort was 10 months (range 3 -20). The survival rate was 85% and 47% at 6
months and 1 year respectively.
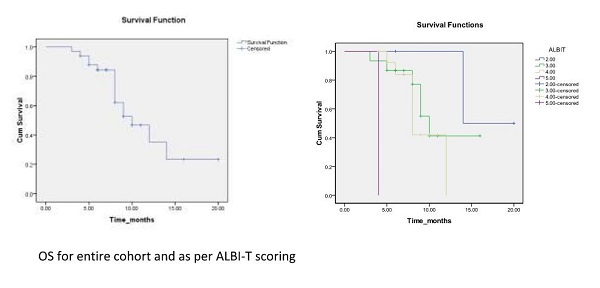
There was a significant survival
difference on sub classifying patients as per ALBI T score. The median OS of
patients with ALBI-T score 2, 3, 4 and 5 is 14, 10, 8 and 4 months respectively (p=0.001).
At 1 year OS for score 2 was 100 % whereas for score 3 and 4 was 42% each
(p=0.001).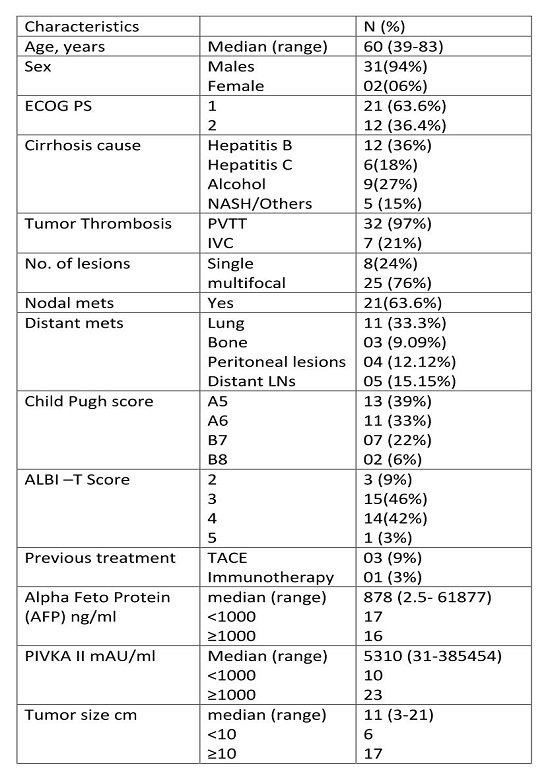
Conclusion
Sub-classification
of BCLC stage C as per ALBI-T scoring system is required to minimize
heterogeneity within the same tumor stage, that will help to predict survival
and to select optimal treatment strategies.