Impact of modern RT in advanced NSCLC: an exploratory real-life investigation from Lombardy
PO-1257
Abstract
Impact of modern RT in advanced NSCLC: an exploratory real-life investigation from Lombardy
Authors: Giulia Corrao1, Matteo Franchi2, Giulia Marvaso1, Mattia Zaffaroni3, Matteo Pepa4, Stefania Volpe1, Maria Giulia Vincini3, Gaia Piperno3, Annamaria Ferrari5, Barbara Alicja Jereczek-Fossa1
1IEO, European Institute of Oncology IRCCS; University of Milan, Division of Radiation Oncology; Department of Oncology and Hematoncology, Milan, Italy; 2University of Milan-Bicocca, Department of Statistics and Quantitative Methods, Milan, Italy; 3IEO, European Institute of Oncology IRCCS, Division of Radiation Oncology, Milan, Italy; 4IEO, European Institute of Oncology IRCCS, Division of Radiation Oncology , Milan, Italy; 5IEO, European Institute of Oncology IRCCS,, Division of Radiation Oncology, Milan, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
Healthcare administrative datasets represent a
valuable source for real-life data analysis. In the current work data on more than 10
million individuals resident in Lombardy Region (Northern Italy) were
considered. Primary aim is to
compare safety and effectiveness in a non-small cell lung
cancer (NSCLC) patients who
received different patterns of first-line systemic therapy with or without RT.
Material and Methods
Diagnostic ICD-9-CM codes were used for
identifying all patients with a new diagnosis of lung cancer between 2012 and
2019. Among these, patients who started a systemic non-chemotherapy as first-line
of treatment for advanced NSCLC alone or in combination with radiotherapy (RT).
Since the same code is applied to NSCLC and SCLC, systemic treatment (ST)
considered were tyrosine-kinase inhibitors (TKI) or pembrolizumab as drugs
exclusively administered in NSCLC. RT treatments were limited to SBRT and IMRT,
in order to select patients with a better expected prognosis. Patients were
followed from the date of first-line treatment start until 31st
December 2020. Overall survival (OS) was estimated by using the Kaplan-Meier
estimator and differences between groups were compared using the log-rank test.
Hazard ratios, along with 95% confidence intervals (CI) were estimated using
Cox proportional hazards models adjusted for sex, age, year of first-line
treatment start and a cancer multimorbidity score. Analyses were stratified by
type of first-line ST and diagnosis of brain metastasis.
Results
During the study period, 95 patients were
treated with ST + RT, and 1873 with ST alone. After a median follow-up of 13.3
months, median OS was 20.2 and 16.8 months (p= .348), respectively, in the two
groups. Among 1714 patients without brain metastasis (BM), similar patterns of OS
were observed in patients treated with ST + RT, as compared to those treated
with ST alone, being the median OS 21.9 and 18.5 months (p= .488),
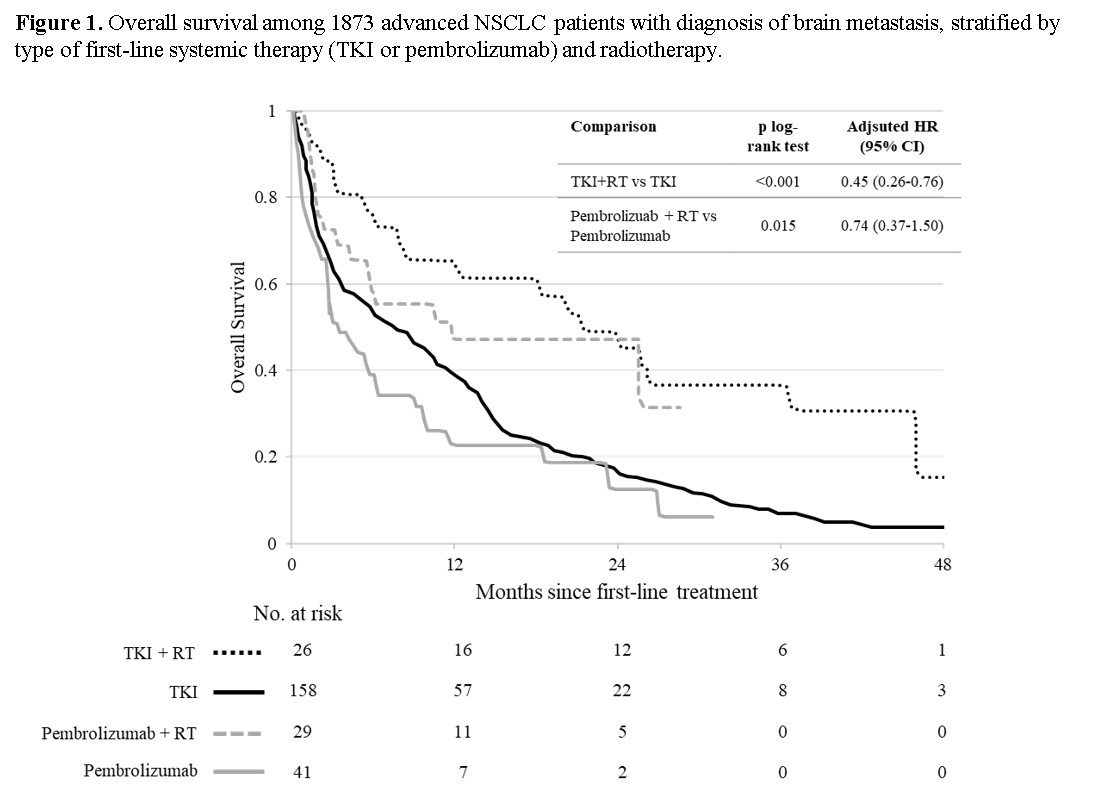
respectively. Conversely, patients with BM (n= 254) treated with RT exhibited a
significant greater OS as compared to those treated with ST alone, being median
OS 21.3 and 7.6 months (p< .001), respectively, corresponding to an adjusted
HR of 0.48 (95% CI 0.32-0.70). This reduction in the risk of death was greater
in patients treated with first-line TKI (adjusted HR= 0.45, 95% CI 0.26-0.76),
while in the smaller sample of those treated with first-line Pembrolizumab was
not statistically significant (adjusted HR=0.74, 95% CI 0.37-1.50) (Figure 1).
Conclusion
Real-world data represent a new reality to
complement evidence from controlled randomized clinical trials. The current
work represent an exploratory study and analyses on the second line therapy
will follow. Our data suggest that in patients with a worse prognosis, as the
ones with BM, RT brings an effective advantage in terms of OS that should be
considered in clinical practice.