Single Institution Experience of Adjuvant Partial Breast Radiotherapy on the Elekta 1.5T MR Linac
Ian Zing Tan,
United Kingdom
PD-0746
Abstract
Single Institution Experience of Adjuvant Partial Breast Radiotherapy on the Elekta 1.5T MR Linac
Authors: Ian Zing Tan1, Adam Mitchell2, Joan Chick2, Jim Sullivan2, Toby Morgan3, Rebekah Lawes4, Trina Herbert4, Helen McNair5, Anna M Kirby5
1Royal Marsden Hospital/ Institute of Cancer Research, Radiotherapy and Imaging, London, United Kingdom; 2Royal Marsden Hospital/ Institute of Cancer Research, Joint Department of Physics, Sutton, United Kingdom; 3Royal Marsden Hospital/ Institute of Cancer Research, Radiotherapy Department, Sutton, United Kingdom; 4Royal Marsden Hospital, Radiotherapy Department, Sutton, United Kingdom; 5Royal Marsden Hospital/ Institute of Cancer Research, Radiotherapy and Imaging, Sutton, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
The MR linac (MRL) heralds a new era of MR guided radiotherapy (RT) for breast cancer. We report our clinical experience on the safety and feasibility of delivering adjuvant partial breast RT on the MRL.
Material and Methods
All breast cancer patients treated within the Prospective Evaluation of Radiotherapy Using Magnetic Resonance Image-Guided Treatment (PERMIT) trial (NCT03727698) were identified. Prior workflow optimization included development of a bolus curtain support designed by the RMH/ ICR workshop to minimise dose deposition to the chin and ipsilateral upper arm from the electron stream effect (Fig. 1). Patient and tumour demographics, RT details, clinical and patient reported outcomes and experience were collected.
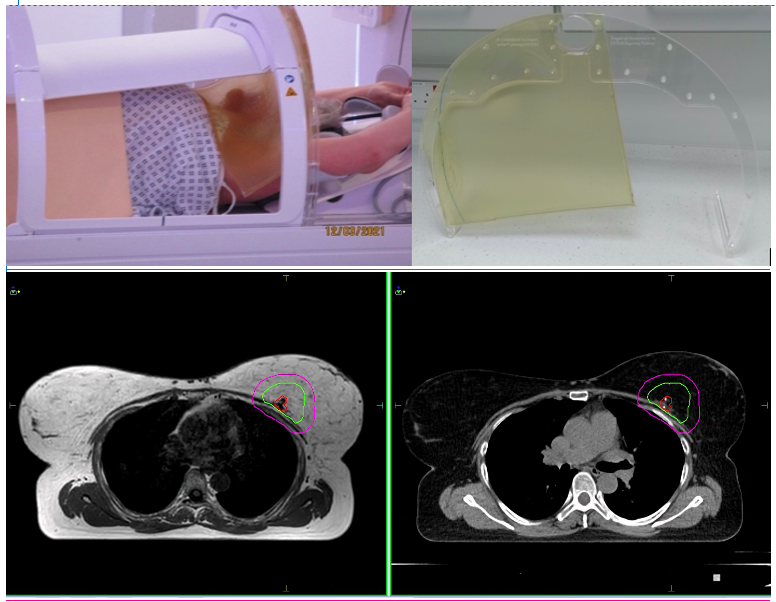
Figure 1 Example of patient setup and bolus support (top images), daily verification MRI (bottom left) and planning CT (bottom right) showing GTV (red), CTV (green) and PTV (pink).
Results
A total of 10 female patients were treated between January 2020 and July 2021. The mean age was 62 (range 51-80) and WHO performance status 0-1. All had histologically proven, completely resected, unifocal T1-2 (<3cm) N0M0 invasive ductal carcinoma with ER+/HER2- without lymphovascular invasion or extensive DCIS. They all underwent breast conserving surgery including sentinel lymph node biopsy and commenced on adjuvant endocrine therapy. A total of 80 fractions (#) were delivered - the first 3 patients received 40Gy/15#/3weeks, while the remaining 7 patients received 26 Gy/5#/1week as per FAST FORWARD. All underwent non-contrast planning CT scans supine on an Elekta WingSTEP M LowTM board. Target volumes and OARs were defined as per IMPORT LOW (Fig. 1). All plans were optimized using Monaco v5.40.01 and all mandatory dose constraints were met. Treatment was delivered using IMRT with 4-6 coplanar 7 MV FFF photon beams following an Adapt to Position workflow. All patients had daily T1-weighted pre-treatment session MRI (T1 3D Tra) and 2D cine-MRI (bFFE Cor Sag Tra RealTime) acquired throughout RT delivery. Five patients in the 5# cohort also had daily post-treatment MRI (T1 3D Tra). The patient setup, RT and post-treatment scan acquisition time was longer in the 5# cohort and resulted in a longer couch time (22+- 2mins) compared to the 15# cohort (16+-2mins). This was under the 30 mins feasibility endpoint (Tab. 1). At baseline, 2 patients had G2 breast pain, while at 2-6 weeks post RT, 1 patient had G2 radiation dermatitis. All toxicities resolved to G0-1 over time, with no ≥G3 toxicities reported. The majority of patients reported RT to be comfortable and tolerable. At their last follow up (range 6 weeks-12 months),all patients were alive with no local or distal recurrences.
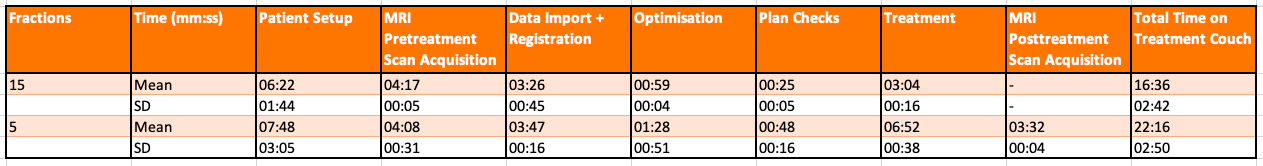 Table 1 Average MRL timings for 5 and 15# cohort.
Table 1 Average MRL timings for 5 and 15# cohort.
Conclusion
Delivery of adjuvant partial breast RT on the MRL is feasible and safe with high patient satisfaction and tolerability. Ongoing work is underway to derive new CTV-PTV margins and explore new indications of breast RT on the MRL.