Late Toxicity and Efficacy of Hypofractionated Prostate RT with Focal Boost in the DELINEATE trial
Julia Murray,
United Kingdom
OC-0106
Abstract
Late Toxicity and Efficacy of Hypofractionated Prostate RT with Focal Boost in the DELINEATE trial
Authors: Julia Murray1, Alison Tree2, Laura Potts3, Ranga Gunapala3, Emily Greenlay3, Emma Alexander4, Annie Gao5, Helen McNair4, Irena Blasiak-Wal6, Aslam Sohaib7, Chris Parker4, Nandita deSouza8, David Dearnaley8
1The Royal Marsden NHS Foundation Trust, Radiotherapy , London, United Kingdom; 2The Royal Marsden NHS Foundation Trust , Radiotherapy , London, United Kingdom; 3The Royal Marsden NHS Foundation Trust, Research and Development , London, United Kingdom; 4The Royal Marsden NHS Foundation Trust, Radiotherapy, London, United Kingdom; 5The Institute of Cancer Research, Bob Champion Unit, London, United Kingdom; 6The Royal Marsden NHS Foundation Trust, Physics, London, United Kingdom; 7The Royal Marsden NHS Foundation Trust, Radiology, London, United Kingdom; 8The Institute of Cancer Research, Radiotherapy and Imaging, London, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
The most common site for local recurrence after prostate radiotherapy (RT) is the dominant intraprostatic tumour lesion (DIL), suggesting that focal radiation boosts to the DIL can improve the therapeutic ratio. As moderate hypofractionation is currently standard of care for localised prostate cancer, we investigated the toxicity and efficacy associated with a dose-escalated image-guided-IMRT boost to the DIL identified on MRI using a 20-fraction schedule.
Material and Methods
Patients with intermediate or high-risk prostate cancer were recruited in a single-institution, prospective phase II trial (ISRCTN04483921). Patients treated within Cohort B of the DELINEATE trial received RT to the whole prostate, to a dose of 60Gy, with a simultaneous integrated boost to the dominant nodules planned to a total dose of 67Gy in 20 fractions. Boost was permitted for no more than 3 separate nodules, and no maximum boost volume was stipulated. Mandatory dose constraints were defined for both target coverage and OARs including rectum, bowel, bladder and urethra. Clinician-reported outcomes (CRO) were assessed using CTCAE v4 and RTOG, with patient-reported outcomes (PRO) including EPIC-26 and IPSS. The primary endpoint was cumulative RTOG late rectal toxicity of grade 2+ at 1 year. We report the primary endpoint, late toxicity to 5 years from start of RT and biochemical relapse-free survival (bRFS) at 5 years.
Results
Between October 2013 and October 2017, 158 patients were recruited. Median follow-up at time of analysis was 63 months. At time of recruitment, median age of included patients was 72 (range: 60-83) years, median PSA of 10.7 (IQR:7.6-15.0) nmmol/l and 49.4% had MRI staged T3 disease. All patients received hormone therapy, with most patients (77.1%) prescribed 6 months LHRHa therapy. Boost prescription dose was achieved for all patients. The cumulative 1-year incidence of RTOG grade 2+ rectal toxicity was 7.2% (1-sided 95% CI: 11.5%). There was no reported late grade 3 or worse rectal toxicity at 1 year. The 5 year late cumulative RTOG grade 2+ GI and GU toxicity rates were 14.6% and 18.2% respectively. Figure 1 displays the grade distribution (%) of rectal and urinary adverse events measured with RTOG and CTCAE and the cumulative incidences. There was a higher prevalence of Grade 1+ GI and GU toxicity assessed using CTCAE than RTOG, with the largest differences for GU toxicity. Frequencies of overall bowel, urinary and sexual bother (EPIC-26) were similar between pre-RT and 2 years from start of RT (Figure 2). Four biochemical failures were seen, with 5-year bRFS of 96.7% (95% CI: 91.3-98.8).
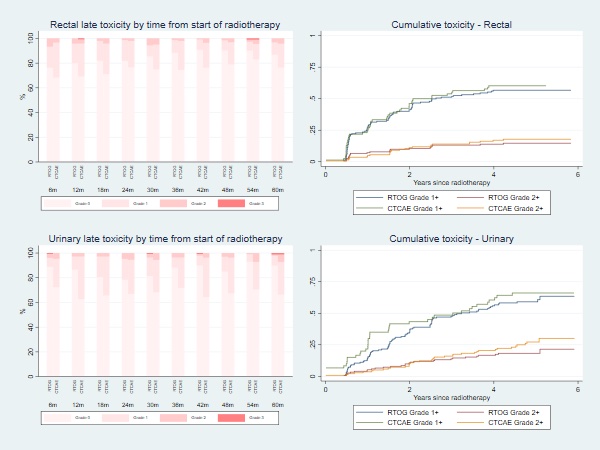
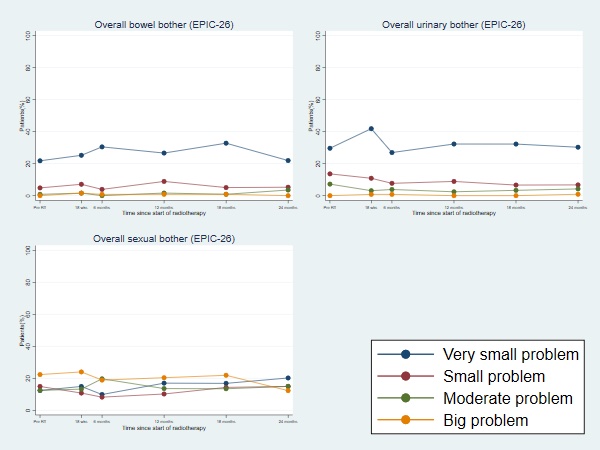
Conclusion
Delivery of a focal boost with moderate hypofractionation is feasible and effective, with CRO and PRO toxicity data comparable to contemporary series without a boost. However, incidence of GU toxicity now has predominance over GI toxicity. The efficacy of a DIL boost using the same dose +/- pelvic RT is under investigation in the UK randomised phase III trial, PIVOTALboost (ISRCTN80146950).