External validation of a prediction model for two-year mortality in esophageal cancer cohorts
Maaike Berbee,
The Netherlands
OC-0109
Abstract
External validation of a prediction model for two-year mortality in esophageal cancer cohorts
Authors: Maaike Berbee1, Christina Muijs2, Francine Voncken3, Leonard Wee1, Edwin Oldehinkel2, Arjen Schaaf van der4, Johannes Reitsma5, Ewoud Schuit6
1Maastro, Radiation Oncology, Maastricht, The Netherlands; 2University Medical Center Groningen, Radiation Oncology, Groningen, The Netherlands; 3The Netherland Cancer Institute/Antoni van Leeuwenhoek, Radiation Oncology, Amsterdam, The Netherlands; 4University Medical Center Groniningen, Radiation Oncology, Groningen, The Netherlands; 5University Medical Center Utrecht, Julius Center, Utrecht, The Netherlands; 6Utrecht University Medical Center, Julius Center, Utrecht, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Although chemo-radiotherapy has been shown to improve the oncological
outcome of esophageal cancer (EC) patients, thoracic radiotherapy may also
cause long term toxicity and increase the risk of non-cancer related death due
to radiation exposure of normal tissues. For lung cancer patients a model to
predict 2-year mortality using GTV volume and mean heart dose (MHD) has been
developed and validated previously (https://nvro.nl/images/documenten/rapporten/LIPP_longen_final_01122019.pdf).
We here aimed to validate this model for EC patients.
Material and Methods
Five EC patient cohorts from 3 different Dutch radiotherapy centers were
used for model validation. Two cohorts of patients (n=170) were used for external
validation in the setting of definitive chemoradiotherapy (dCRT) and 3 cohorts
(n=568) for validation in the neo-adjuvant setting (nCRT). External validity was assessed in
terms of calibration (i.e., agreement between predicted and observed risk) by
calibration plots, and discrimination (i.e., ability of the model to distinguish
between those who died within 2 years and those who did not) by assessment of
the c-statistic. If indicated, the model was updated by adjustment of the
intercept, or intercept and slope (“recalibration”), or regression coefficients
(“revision”) of the model. An additional logistic regression analysis assessed
the confounder-adjusted association between the MHD and the outcome as well as
the incremental value of the MHD on top of other known predictors of 2-year
mortality (GTV, age, gender, histology, N stage, WHO performance status and
tumor length). The incremental value was assessed using a likelihood ratio test
(LRT) and the integrated discrimination index (IDI).
Results
For the dCRT patients the model showed good calibration after adjustment
of the intercept (Figure 1). The c-statistic was 0.67 (95%CI: 0.58 to 0.75).
For nCRT group the model needed recalibration (Figure 2). The c-statistic of
the recalibrated model equaled 0.62 (95%CI: 0.57 to 0.67). The additional
analysis showed that the MHD was independently associated with 2-year mortality
(OR = 1.31; 95% CI 1.03-1.61) and had added value on top of other prognostic
factors (LRT p-value = 0.018 and IDI 0.0073; 95%CI 0.0012-0.13; p-value = 0.019).
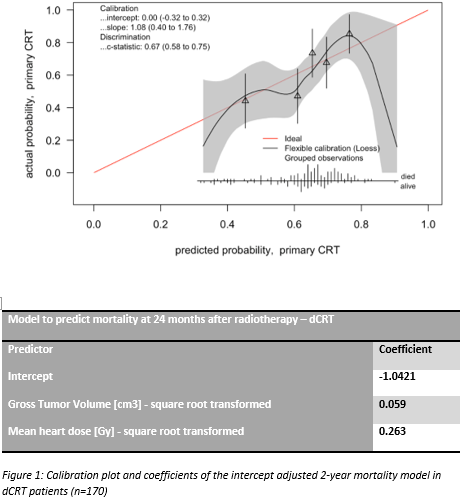
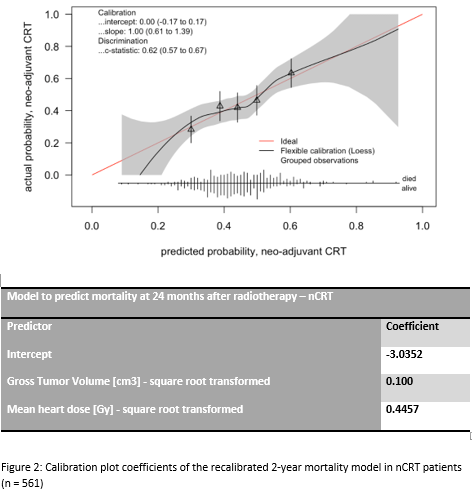
Conclusion
An existing prediction model for 2-year mortality in lung cancer
patients, based on the predictive factors GTV and MHD, was externally validated
in esophageal cancer patient cohorts. Separate updated models for dCRT and nCRT
in esophageal cancer were developed. The additional analyses support the existence of an independent effect of MHD on 2-year
mortality.