QuADRANT-a Multidisciplinary EU Project Aiming to Increase Uptake and Utilisation of Clinical Audit
PO-1052
Abstract
QuADRANT-a Multidisciplinary EU Project Aiming to Increase Uptake and Utilisation of Clinical Audit
Authors: Núria Jornet1, Francesco Giammarile2, Primoz Strojan3, Mary Coffey4, Adrian Brady5, Jonathan Clark6, Monika Hierath6, David Howlett7
1hospital de la Santa Creu i Sant Pau, radiofisica i radioprotecció, Barcelona, Spain; 2Centre Léon-Bérard, Nuclear Medicine, Lyon, France; 3Institute of Oncology, Radiation Oncology, Ljubljana, Slovenia; 4Trinity Centre for Health Sciences, Radiation therapy, Dublin, Ireland; 5Mercy University Hospital, Radiology, Cork, Ireland; 6ESR, European and International Affairs , Vienna, Austria; 7ESR, ESR Audit and Standards Sub-Committee, Vienna, Austria
Show Affiliations
Hide Affiliations
Purpose or Objective
QuADRANT is a three-years project established on behalf of the European Commission to review the status of clinical audit across Europe, to identify good practices and produce guidelines and recommendations to improve clinical audit implementation in the field of radiation medicine. QuADRANT is led by the European Society of Radiology, ESR working with ESTRO and the European Association of Nuclear Medicine, EANM. A core part of the project is a pan European survey on clinical audit practice. We present the main findings of this survey.
Material and Methods
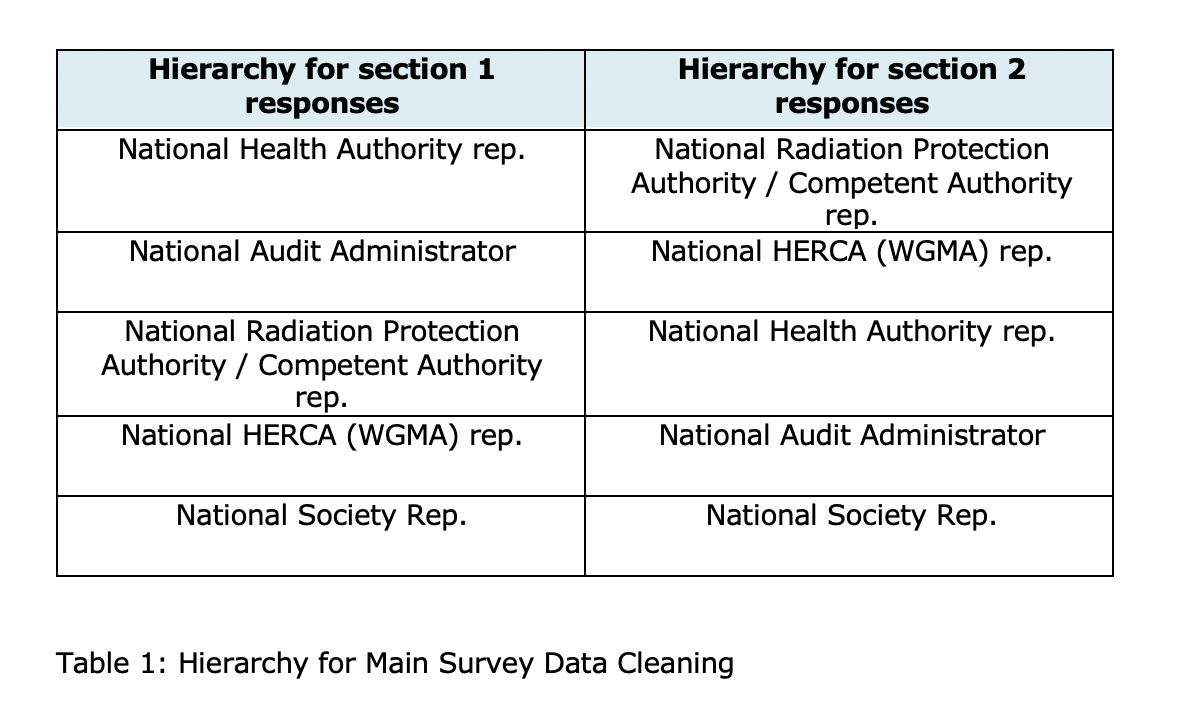
The survey was developed over a period of three months with several iterations and incorporating feedback from experts within the project Steering Group, Advisory Board, consortium members, and from the European Commission. It comprised 2 sections and 35 questions. The larger first section covered clinical audit practice and infrastructure, the second section focussing on clinical audit and the Basic Safety Standards (BSSD). It was launched on March 2021 to national health authorities, Radioprotection competent authorities and national professional societies. The survey was closed on May 2021. In case of conflicting answers between respondents from the same country a complementary approach, then a ‘hierarchy’ was adopted (table 1).
Results
In total, 104 responses to the survey were recorded. Responses providing no answers to questions or duplicate were removed. Thus, 83 responses from EU27+4 countries were considered in the analysis. Within the respondents there were 12 national health authority and 28 radiation competent authority representatives. Most countries reported a central body responsible for audit policy/development of quality indicators/data collection. Only a minority described available national clinical audit manuals/guidance. There is considerable variation in the types of clinical audit occurring between countries, with a significant minority of countries declaring no organised national clinical audit activity. Hospital network collaboration or national professional society involvement were rarely reported. Barriers to effective clinical audit were: insufficient funding (at several levels), low national priority (which is likely to be part of the reason for the insufficient funding), lack of time and staff. Regarding incentives, 37% of respondents indicated that there were none, showing a lack of appreciation of the mandatory legal requirements.
Conclusion
QuADRANT has identified wide variation across Europe in the uptake and implementation of clinical audit in both clinical practice and as mandated within the BSSD. The solutions to the issues raised are complex and multifactorial but will require effective co-operation and collaboration by key European governmental stakeholder and healthcare organisations. The national professional societies will have a crucial role in this process.