Patients with early stage breast cancer or intermediate-risk
prostate cancer were selected for study.
After signing the informed consent form,
simulation, volume delimitation and dose prescription were performed. The
treatment schedule for breast cancer patients was 40.05 Gy on the breast and 48
Gy on the lumpectomy bed in 15 fractions (integrated boost IMRT) and for
prostate cancer patients, 60 Gy in 20 fractions.
An optimized dosimetric study was performed for
the two accelerators and the dose-volume histograms were compared. The patient
was eligible if the histograms did not show significant differences neither in
the prescribed dose nor in the organs at risk according to the usual procedures.
Half of the patients underwent the treatment in
a Truebeam© and the other half in a Halcyon®. The distribution was randomized
by unit and stratify by tumour site. In session number 8 (breast patients) and
number 11 (prostate patients) the treatment unit was changed to the other linac.
Treatment was performed daily with IGRT and online correction in both units.
Two different questionnaires have been designed
to determine the patients' experience with the treatment team, following the
recommendations of the scale defined by Mastaglia(1). Questionnaire 1 assesses
the treatment unit and is answered in the 5th session of each unit (session 5th
for the first linac and 13th-breast- or 16th-prostate- for the second linac).
Questionnaire 2 allows comparison between the linacs and is answered on the
last day of treatment (Table 1).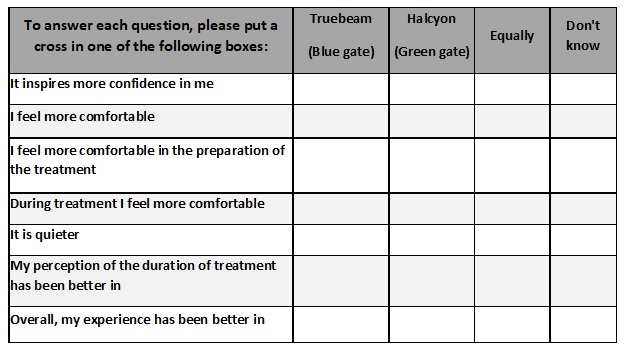
(1) Barbara Mastaglia, Christine Toye and Linda J. Kristjanson.
Ensuring content validity in instrument development: Challenges and innovative
approaches, Contemporary Nurse, 14:3, 281-291, DOI: 10.5172/conu.14.3.281
(