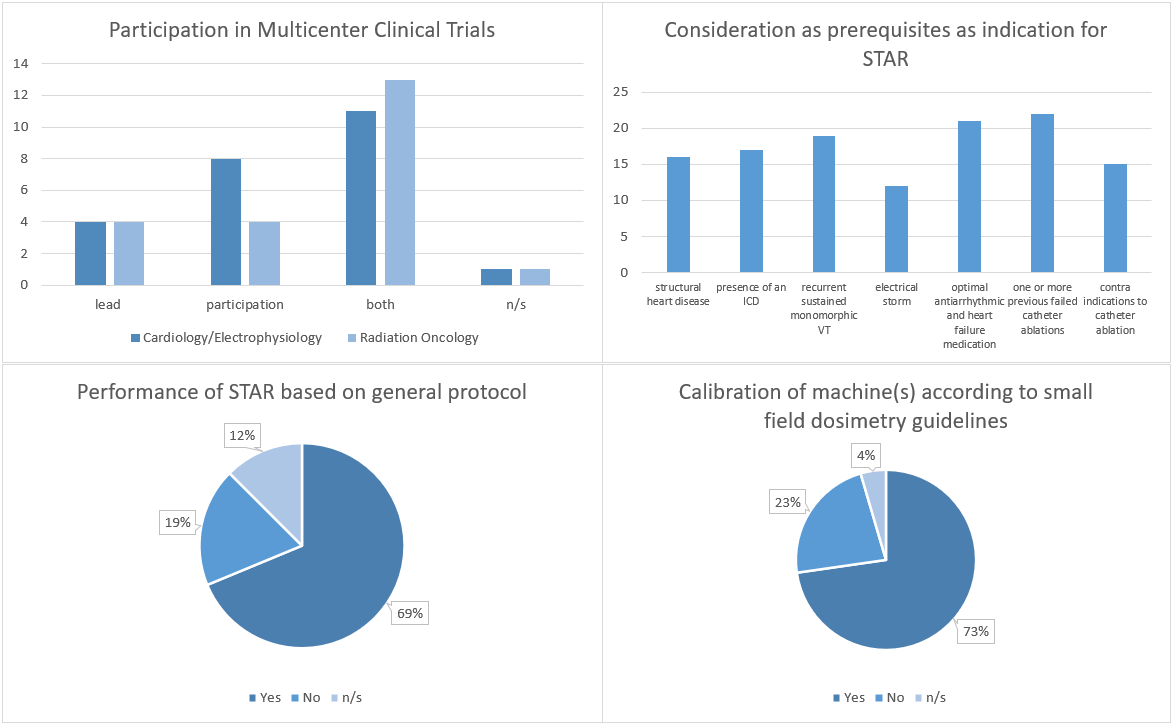
All clinics
participating in STOPSTORM completed the survey. Catheter ablation for VT (20/24 centres ≥ 20 p.a.) and SBRT (13/22 centres
> 200 p.a.) experience is high and technical equipment is state-of-the-art. 16 centres performed a total of 84 STAR
treatments until May 2021 and 11 centres already participate in clinical trials
for STAR.
Target
volume definition is based on electroanatomical mapping during VT (23), pace
mapping (18), reduced voltage areas (15) and/or late ventricular potentials (18).
Half of the centres therefore include the clinical VT areas, while the other half
include the whole arrhythmogenic substrate.
18 and 4 centres use 4D-CT-ITV or
tracking/gating concepts for respiratory and 10 vs. 10 centres use ECG-triggered-4D-CT or statistical margins
for cardiac motion management, respectively. In all but one clinic, a dose of
25 Gy in a single fraction is applied. The prescription method, inhomogeneity
in the PTV and planning technique however varies greatly.
13/22 centres report technical system
audits every 1-2 years, while regular dose audits are performed in 16/22 centres.
17/22 centres perform
end-to-end tests specifically for SBRT. All centres perform patient-specific
plan verifications for STAR, but with various evaluation criteria.
A summary
of various survey results is given in figure 1.