A Registry for Analysis of Data to Advance Personalised Therapy with MR-Linac (ADAPT-MRL)
Vikneswary Batumalai,
Australia
PO-1064
Abstract
A Registry for Analysis of Data to Advance Personalised Therapy with MR-Linac (ADAPT-MRL)
Authors: Michael Jameson1, Vikneswary Batumalai1, Amanda Woods2, Tania Twentyman1, Vicki Sproule2, Joseph Christiansen3, Neil Kennedy3, Maria Marney1, Kris Barooshian4, Michael Plit5, Jayd Lynch2, Raj Jagavkar1, Helen Ormandy6, John Christodouleas7, Florian Pietzsch5, Jeremiah de Leon1, Patrick Foley5
1GenesisCare, Oncology, Sydney, Australia; 2GenesisCare, Oncology, Newcastle, Australia; 3GenesisCare, Innovations, Sydney, Australia; 4GenesisCare, Insights, Sydney, Australia; 5GenesisCare, CRO, Sydney, Australia; 6GenesisCare, CRO, Melbourne, Australia; 7Elekta AB, Medical Affairs & Clinical Research, Atlanta, USA
Show Affiliations
Hide Affiliations
Purpose or Objective
The
pairing of magnetic resonance imaging (MRI) technology with the linear
accelerator is an important recent innovation. The MR-Linac allows
visualisation of anatomical and functional changes during radiotherapy (RT) and
adapts the treatment to achieve optimal therapy. While the MR-Linac offers the
promise of high precision treatment, it is important to evaluate this novel
technology to understand the clinical outcomes achieved in the short and long
term. Analysis of Data to Advance Personalised Therapy with MR-Linac
(ADAPT-MRL) is a multi-site, multinational, observational cohort registry
designed to collect data on the use of MR-Linac for RT and patient outcomes.
The registry will provide a linked repository of technical and clinical data
that will form a platform for prospective studies and technology assessment.
Material and Methods
Ethics
approval was granted by St Vincent’s Hospital Human Research Ethics Committee. Figure
1 shows the design of the ADAPT-MRL registry. This registry aims to include an
estimated 10,000 eligible participants across Australia and other countries
over a 7- to 10-year period. Participants will undergo treatment and
assessments in accordance with standard practice. Toxicity and survival
outcomes will be assessed at baseline, during treatment, and with 3 monthly
follow-up until 24 months, patient reported outcome measures will also be
collected. Participants with a variety of cancers will be included.
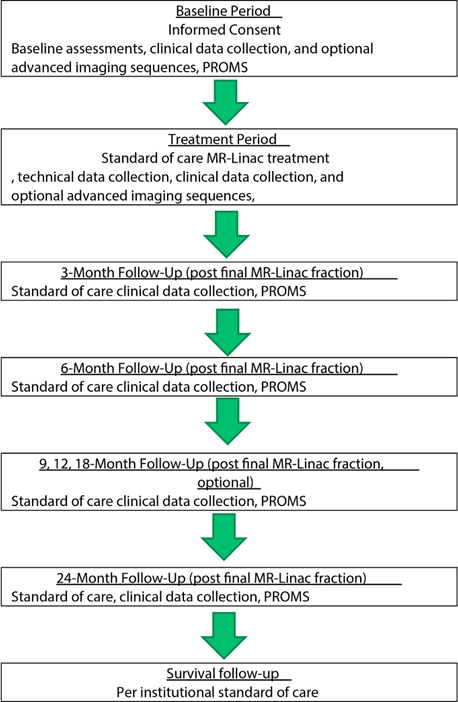
Figure 1: Design of the ADAPT-MRL registry
Results
In
2020, the ADAPT-MRL registry was established in coordination with investigators
of The MOMENTUM Study [1], another multi-institutional observational registry
of MR-Linac treatments, to enable efficient data aggregation and coordinated
sub-studies. Since then, 79 patients have consented to participate in the
registry and 41 patients have completed 3-month follow-up.
Conclusion
Data
obtained from the ADAPT-MRL registry is expected to provide evidence on the
safety and efficacy of the MR-Linac, a new technical innovation in radiation
oncology. We expect this registry will generate data that will be used to
optimise treatment techniques, MR-Linac software algorithms, evaluate
participants’ outcomes and toxicities and to create a repository of adapted
plans, anatomical and functional MR sequences linked to participants’ outcomes.
[1]
Sophie R, Christodouleas JP, Blezer EL, Akhiat H, Brown K, Choudhury A, Eggert
D, Erickson BA, Faivre-Finn C, Fuller CD, Goldwein J. The MOMENTUM Study: An
international registry for the evidence-based introduction of MR-guided
adaptive therapy. Frontiers in Oncology. 2020;10.