Stereotactic MR guided online adaptive radiotherapy for abdominal and pelvic lymph node metastases
Kasia Owczarczyk,
United Kingdom
PD-0502
Abstract
Stereotactic MR guided online adaptive radiotherapy for abdominal and pelvic lymph node metastases
Authors: Kasia Owczarczyk1, Hannah Harford-Wright1, Sara Shergill1, Tim Sevitt1, Jane Lynch1, Judy Harris1, Ben George2, Andy Gaya1, James Good2
1GenesisCare UK, Centre for Radiotherapy at Cromwell Hospital, London, United Kingdom; 2GenesisCare UK, Centre for Radiotherapy at University Oxford Hospital, Oxford, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
Stereotactic MR guided online adaptive
radiotherapy (SMART) offers significant dosimetric advantages in the treatment
of abdominal and pelvic malignancies due to complex surrounding anatomy and
mobile organs at risk1,2 Here, we report patient tolerability, acute
toxicity and dosimetric outcomes of SMART in a cohort of patients with abdominal
and pelvic lymph node metastases.
Material and Methods
Patients who underwent SMART between
01.01.2020 and 31.09.2021 were retrospectively included. Acute toxicity was prospectively
assessed using Common Terminology Criteria for Adverse Events (CTCAE) v4.0.
Dose-volume histograms (DVHs) for target
volumes and OARs were obtained for every treatment course at the following time
points:
1. Baseline: simulation plan applied to
anatomy at simulation
2. Pre-adaptation: simulation plan applied to
the anatomy of the day prior to each treatment fraction
3. Post-adaptation: re-optimized plan applied
to the anatomy of the day prior to each treatment fraction
Target volume coverage and organ-at-risk violation metrics for initial non-adaptive and adaptive plans were compared using non-parametric Dunn method with
Bonferroni correction for multiple comparisons.
Results
N=15 patients (median age 58, range 39-76, 7/15
females (47%)) with oligometastatic lymph nodes (coeliac, pre-sacral,
aortocaval, porta hepatis, mesenteric, common iliac and peri-pancreatic) were
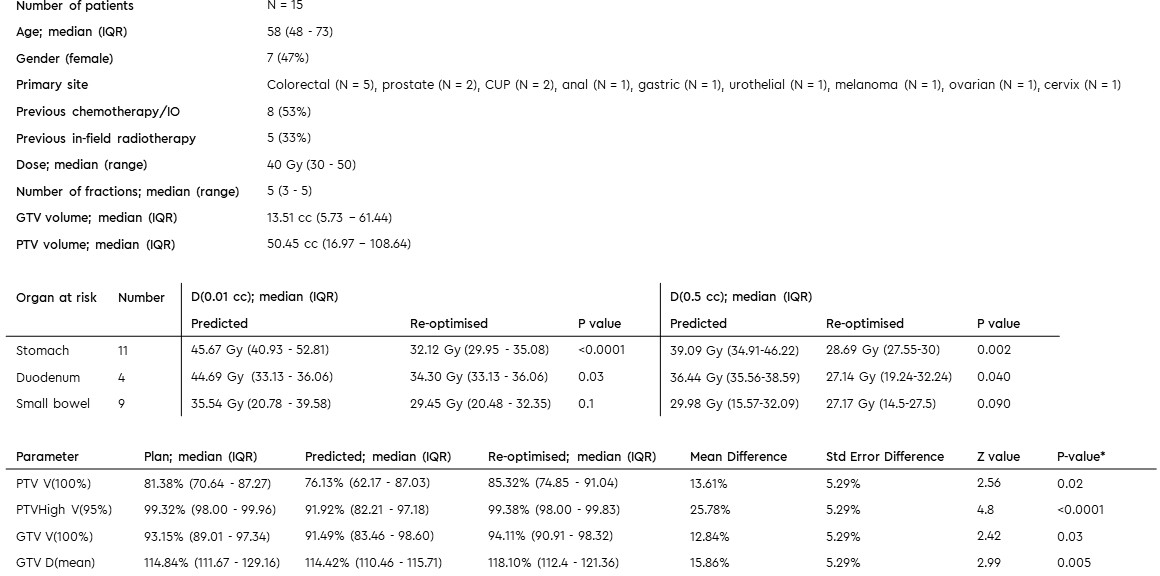
included (Table 1).
No CTCAE v4 G3 or higher toxicities were
recorded on-treat or at two-week post treatment review.
Median GTV and PTV volumes on reference plan
were 13.51cc (IQR 5.73-61.44) and 50.45 (IQR 16.97-108.64) respectively. Median
dose prescription was 40Gy (range 30-50Gy) in 3-5 fractions. All initial plans
met hard OAR constraints.
In total, 62 fractions were delivered, using
daily adaptive plans with data available for 55 fractions. Target re-contouring resulted in median 0.24% (IQR -2.68
– 3.1%) volume change in GTV. There were no OAR violations on the base
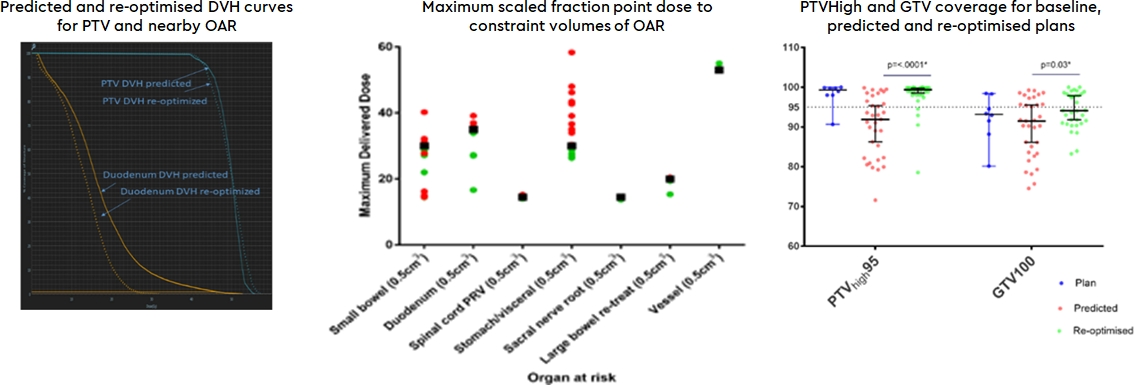
plans. On pre-adaptation plans, OAR violation occurred in 37/55 (67.27%) of fractions versus 5/55 on
post-adaptation plans (9.09%; p<.0001**; Figure 1) Re-optimisation achieved
a mean dose reduction to duodenum and stomach within 3cm of PTV of 27.46% (95%CI 33.34-21.57) and 28.03% (95%CI 36.24-19.82) in D(0.01cc) and D(0.5cc), respectively, whilst significantly improving PTV100, PTV95
and GTV100 coverage as well as GTVmean dose (Table 1, Figure 1).
Conclusion
Our data confirms findings from similar
series3 that SMART is well tolerated, safe and
offers a favourable dosimetric profile and an improved therapeutic index in
abdominal and pelvic sites