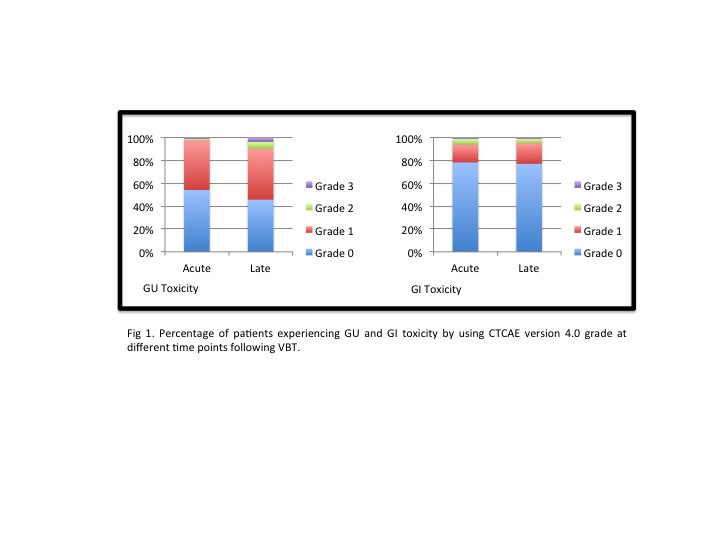
Median age was 68. Thirty-five patients (42,2%) were FIGO Stage IA and 48 (57,8%) stage IB. With a median follow-up of 36 months acute GI and GU grade 1 toxicity was presented in 13 patients (15,7%) and 36 (43,4%), and grade 2 in 4 patients (4,8%) and 1 (1,2%) respectively. One patient (1,2%) presented grade 3 acute GI toxicity (fecal urgency due to proctitis) and 2 patients (3,6%) developed grade 3 acute GU toxicity (urinary incontinence). Late GI and GU grade 1 toxicity was presented in 15 patients (18, 7%) and 37 (44, 6%), and grade 2 in 2 patients (3,6%) and 5 (6%) respectively. One patient (1, 2%) had grade 3 late GU toxicity (urinary incontinence) (Fig 1). Grade 1 vaginal stenosis was developed in 16 patients (19,3%) and grade 2 in 1 (1,2%). Four patients presented (4,9%) chronic dyspareunia. Median Dmax and D2cc in rectum were 6.43Gy (IQR: 5,54- 7,14) and 4,46 (IQR: 3,71- 5,01) respectively, and median Dmax and D2cc in bladder were 6.68Gy (IQR: 6,05-7,47) and 5.29 (IQR: 4,70-5,68).
Statistically significant association was observed in both acute (p=0.048) and late (p= 0.05) GU toxicity with bladder Dmax > 7.46Gy and D2cc > 5.67Gy. Patients who presented grade 3 GU toxicity were in the highest Dmax and D2cc quartile. No significant association was observed in acute or late GI toxicity with rectal doses.
Local and distant recurrence rate was 3,6% and 4,8% respectively, and overall survival was 91%.