Durvalumab with cetuximab and radiotherapy for locally advanced HNSCC: a phase 1/2 trial
PO-0150
Abstract
Durvalumab with cetuximab and radiotherapy for locally advanced HNSCC: a phase 1/2 trial
Authors: Pierluigi Bonomo1, Isacco Desideri1, Monica Mangoni1, Calogero Saieva2, Carlotta Becherini1, Mauro Loi1, Cecilia Cerbai1, Michele Ganovelli1, Viola Salvestrini1, Giulia Stocchi1, Annarita Palomba3, Margherita Zani4, Lorenzo Livi1
1Azienda Ospedaliero-Universitaria Careggi, Radiation Oncology, Florence, Italy; 2 Institute for cancer research, prevention and clinical network (ISPRO), Cancer Risk Factors and Lifestyle Epidemiology Unit, Florence, Italy; 3 Azienda Ospedaliero-Universitaria Careggi, Section of Pathological Anatomy, Department of Health Sciences, Florence, Italy; 4Azienda Ospedaliero-Universitaria Careggi, Medical Physics, Florence, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
To report on the anti-tumor activity of a novel combination consisting of Durvalumab (DUR), Cetuximab (CTX) and Radiotherapy (RT) in high-risk locally advanced head and neck squamous cell carcinoma (HNSCC).
Material and Methods
DUCRO (NCT03051906) was designed as a multi-center, single-arm, phase I/II study. Patients affected by high-risk (>N2a or>T3, any N) larynx, hypopharynx and HPV negative oropharynx or HPV-positive oropharynx (>T2, >N2b, >10 pack/years; all staged with TNM/AJCC 7thedition) were eligible. The primary endpoint of the study was 2-year progression-free survival (PFS). A safety run-in was planned. Secondary endpoints were tolerability, LRC, OS, and HRQoL. In order to avoid the potential threat of chronic locoregional lymphodepletion, a selective sparing of lymph node levels in the elective volume by keeping a mean dose below 40 Gy was outlined in the protocol. The experimental treatment consisted of IMRT (69.9 Gy/2.12 Gy in 33 fractions) with concurrent CTX (400 mg/m21 week before RT start followed by 250 mg/m2 weekly) and DUR (fixed dose of 1500 mg every 4 weeks starting from RT-CTX week 1) followed by adjuvant DUR (to a maximum of 6months after completion of RT-CTX). Due to regulatory issues which prevented from opening multiple centers, COVID-19 pandemic and DUR withdrawal from supporting company, the study was prematurely terminated in 04/21.
Results
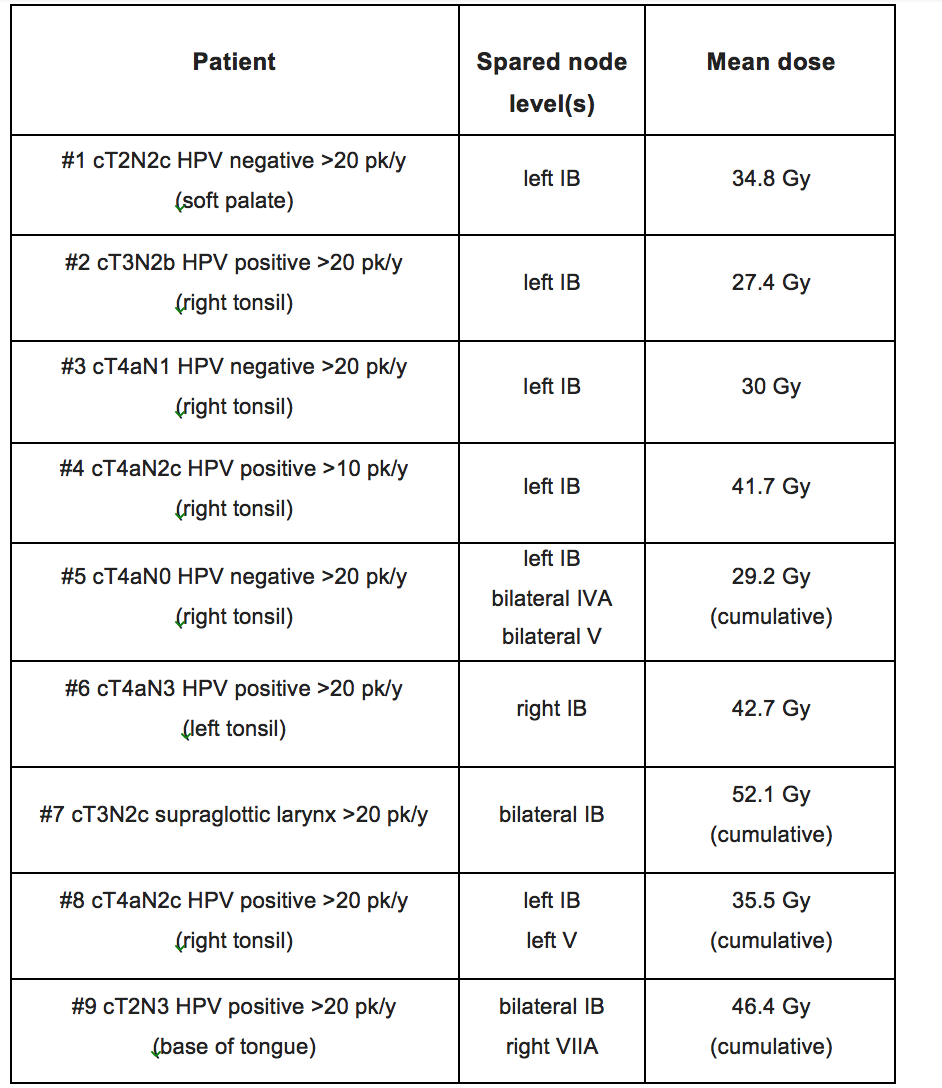
Between July 2019 and August 2020, 9 patients were enrolled in the study. At diagnosis, the median age of the population was 68 years (range, 57-75). All patients were male, and the predominant primary tumor site was the oropharynx (88.8%), of whom 5/8 had HPV positive disease. The vast majority had stage IVA disease, and had significant tobacco exposure (>20 pack/years in 88.8% of them). All tumors had a PD-L1 Combined Positive Score > 1. Optimal drug exposure was observed, with mean relative dose intensity of 85.5% and 87.5% for CTX and DUR, respectively. No radiation breaks were necessary. A grade 4 mucositis lasting for 14 days corresponded to the only dose limiting toxicity we reported (figure 1: in the upper left corner, severe mucositis which persisted for 15 days prompting hospitalization and PEG placement (panel a); from the upper right corner to the bottom left, clockwise, panels b-c-d, slow healing process which occurred over one month). At a median follow-up of 11.5 months (IQR 7.7-16.7) all surviving patients (6 out of 9) are disease-free, with 1 and 2-year PFS rates of 77.7% and 58.3%, respectively. Only one patient died of disease progression. A selective sparing of node levels in the elective volume was performed in all cases, yielding a cumulative mean dose of 37.6 Gy (SD 8.4) (figure 2).
Figure 1
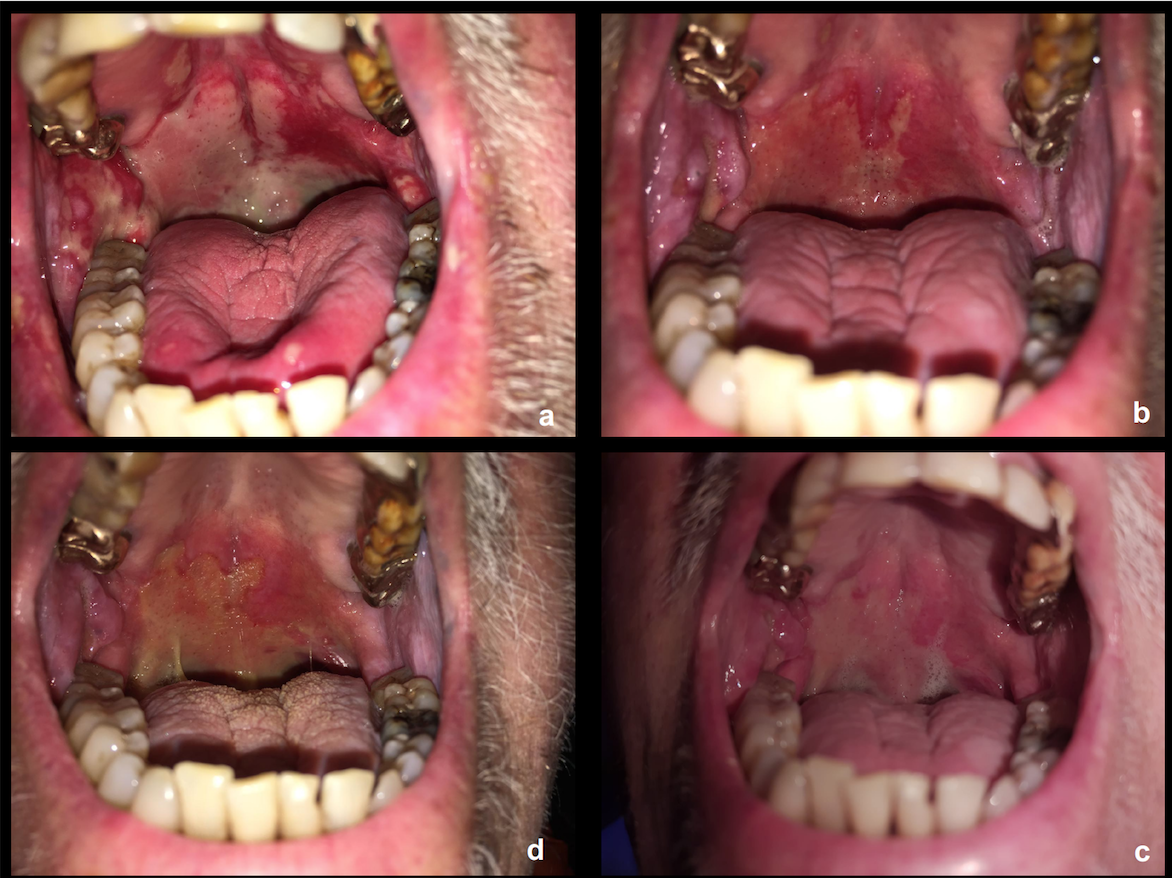
Figure 2
Conclusion
Albeit limited by the small sample size, our preliminary observation of anti-tumor activity and tolerability of DUR in addition to CTX and radiation may warrant further investigations