Feasibility and compliance of StrataXRT in treatment of radiation dermatitis in head and neck cancer
PO-1220
Abstract
Feasibility and compliance of StrataXRT in treatment of radiation dermatitis in head and neck cancer
Authors: Gemma Mckee1, Lucy Rutten1, Peter Larsen2, Aidan Leong2,3
1Wellington Blood and Cancer Centre, Radiation Therapy, Wellington, New Zealand; 2University of Otago, Department of Radiation Therapy, Wellington, New Zealand; 3Bowen Icon Cancer Centre, Radiation Therapy , Wellington, New Zealand
Show Affiliations
Hide Affiliations
Purpose or Objective
The aim of this study was to evaluate the feasibility of Strata XRT (SXRT) in the treatment of radiation dermatitis in head and neck cancer (HNC) patients. SXRT tolerability, compliance and usage were assessed alongside clinician, radiation therapist (RT), and patient perspectives on toxicity. Comparison was against a historical control cohort managed using Fatty E cream (FEC).
Material and Methods
Patients with a diagnosed HNC, aged 18 years or older and prescribed ≥45Gy were eligible for this study. An adjusted version of the Radiation Induced Skin Reaction Assessment Scale (aRISRAS) was developed to capture patient experience and utilisation of SXRT in addition to RT-reported dermatitis and patient-reported impact of skin reaction. RTs were trained in aRISRAS scoring, and assessed for consistency amongst RTs and against clinicians. Dermatitis was scored by clinicians using CTCAE. Assessments were performed weekly from the start of treatment through to one-week following treatment completion.
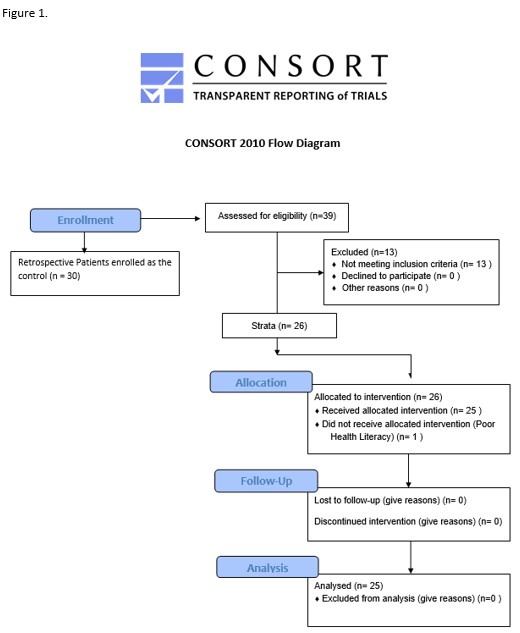
Results
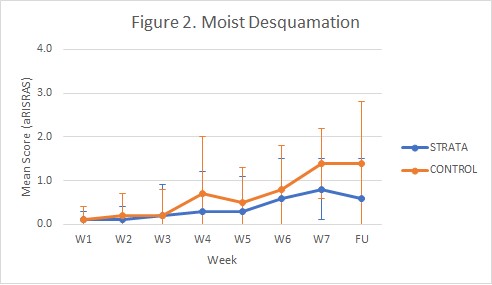
26 patients were allocated to receive SXRT, of whom 25 completed treatment using the product (Fig 1). The FEC control cohort consisted of 30 patients. Patient experience reported SXRT as easy to apply, with stickiness noted frequently and occasional discomfort. Correct application of SXRT (amount and area) was noted in 94% of interactions. Assessment completion rates were 98% and 41% for RTs and clinicians, respectively. No clear distinctions were demonstrated in toxicity between the groups, though aRISRAS trends suggested a reduced incidence of Gr3+ moist desquamation with SXRT compared to FEC (8% vs 23%, respectively) and faster recovery of skin reaction post-treatment with SXRT (Fig 2). Mean (range) utilisation of SXRT was 2.2 (1-4) 50g tubes per patient.
Conclusion
This study confirms feasibility of SXRT in the management of radiation dermatitis for HNC patients. Further investigation is warranted to better define cost-benefit. RT involvement in standardised toxicity assessment enables high quality data to be consistently collected for the benchmarking of clinical outcomes.