Radiation dose response of bone marrow during chemoRT for NSCLC measured by serial FLT-PET
Michael MacManus,
Australia
PD-0971
Abstract
Radiation dose response of bone marrow during chemoRT for NSCLC measured by serial FLT-PET
Authors: Elizabeth Prins1, Michael MacManus1,2, Fiona Hegi-Johnson1,2, Jason Callahan3, Jing Xie4, Rod Hicks5,2, Tim Akhurst6,2, Sarah Everitt1,2
1Peter MacCallum Cancer Centre, Radiation Oncology, Melbourne, Australia; 2University of Melbourne, Sir Peter MacCallum Department of Oncology, Melbourne, Australia; 3Melbourne Theranostics Innovation Centre, Nuclear Medicine Technology, Melbourne, Australia; 4Peter MacCallum Cancer Centre, Centre for Biostatistics and Clinical Trials, Melbourne, Australia; 5Peter MacCallum Cancer Centre, Nuclear Medicine, Melbourne, Australia; 6Peter MacCallum Cancer Centre, Molecular Imaging and Therapeutic Nuclear Medicine, Melbourne, Australia
Show Affiliations
Hide Affiliations
Purpose or Objective
Immune checkpoint immunotherapy improves survival after chemoradiotherapy (chemoRT) in non-small-cell lung cancer (NSCLC). Effective immunotherapy requires functioning adaptive immunity and radiotherapy causes acute marrow injury, potentially reducing efficacy of subsequent immunotherapy. FLT-PET can sensitively image bone marrow proliferation. We analysed serial FLT scans obtained before and during chemoRT as part of a prospective clinical trial.
Material and Methods
Sixty patients were recruited to an observational trial of serial FLT-PET scans in patients with locoregionally advanced NSCLC treated with 3DCRT-planned radical chemoRT (60 Gy with concurrent cisplatin/etoposide or carboplatin/taxol). Complete PET datasets were available for 28 patients. Baseline, week 2, and week 4 FLT-PET scans were retrospectively analysed, using MIM software to segment the skeleton and quantify FLT uptake in defined volumes of interest (VOIs). Dose bins were generated, with isodose lines from 0 Gy up to >5.0 Gy converted to contours to represent VOIs. SUVmax values were calculated in each VOI. Additionally, visual analysis was used to assess the radiation dose at which bone marrow uptake was unapparent at week 2 and 4, representing the lowest radiation dose resulting in ablation for each patient at each time point. Unirradiated bone marrow in a vertebral body represented normal uptake. SUVmax values were normalised to the visual ablative dose, negating the effects of blood pool and isolated voxels with high SUVmax. Data were normalised for each patient and week to represent 0-100% of baseline marrow proliferation.
Results
Dose-response curves were produced illustrating the relationship between radiation dose and FLT SUVmax as an estimate of bone marrow function at each time point for each patient. Individual untransformed patient data are shown in figure 1. There was a significant negative correlation between radiation dose and marrow proliferation from baseline to weeks 2 and 4 (p<0.001). A steeper negative relationship was observed in week 2 compared to week 4, likely reflecting the smaller dose per fraction at each isodose. Transformed curves were also produced, using visual estimates of the marrow ablation dose to represent 0% proliferation (figure 2), also showing a negative correlation between dose and proliferation (p<0.001). At doses ≥3-4 Gy, marrow proliferation was absent at week 2 in all but one patient.
Figure 1
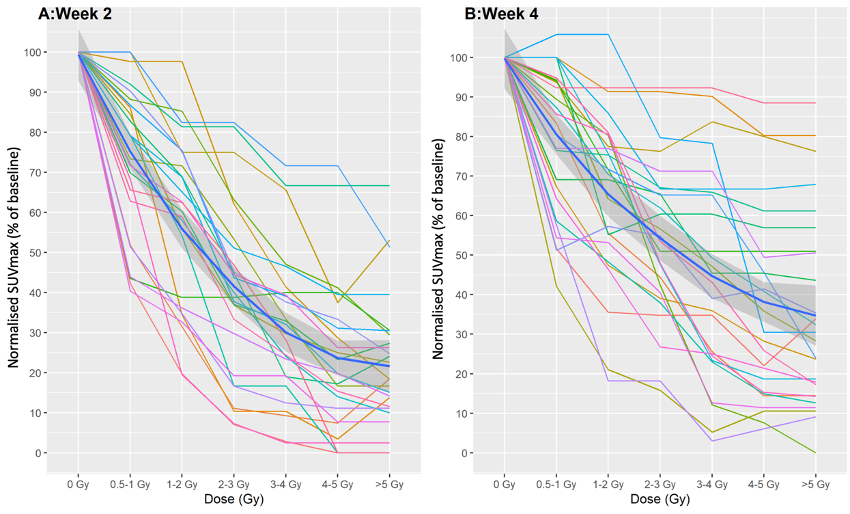
Figure 2
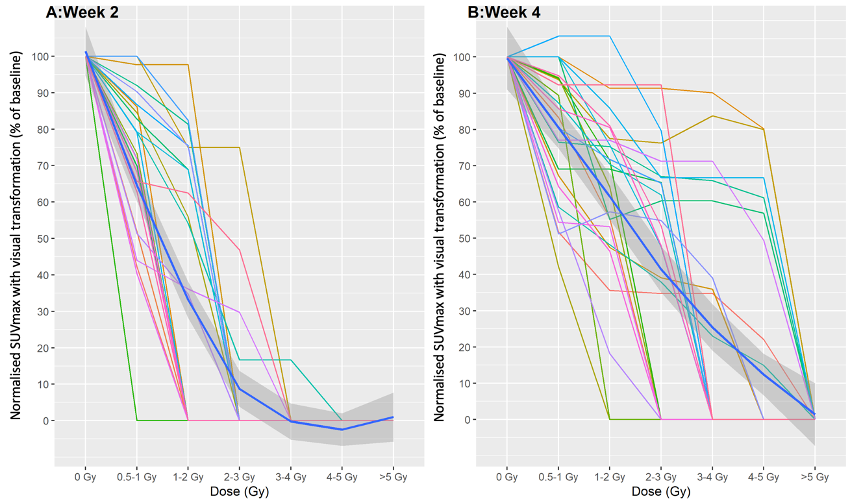
Conclusion
In chemoRT patients, bone marrow proliferation decreased steeply with increasing local radiation doses in weeks 2 and 4 of treatment. The decline was steeper in week 2. Bone marrow proliferation in chemoRT patients was exquisitely sensitive to low doses of radiation, with marrow signal becoming visually-ablated at doses in the 3-4 Gy range for almost all patients at week 2, although with significant individual variation between cases. These data indicate near universal within-field bone marrow ablation during chemoRT.