Time evolved hypoxia guided proton therapy dose painting in HNSCC patients
Shubhangi Makkar,
Switzerland
MO-0956
Abstract
Time evolved hypoxia guided proton therapy dose painting in HNSCC patients
Authors: Shubhangi Makkar1,2, Anne-Sophie Bogaert1, Barbara Bachtiary1, Claudio De Angelis3, Catharina M.L. Zegers4, Erik Roelofs4, Keegan McNamara1,2, Jan Hrbacek1, Damien C. Weber3,5,6, Antony Lomax1,2, Carla Winterhalter1,2
1Paul Scherrer Institute, Center for Proton Therapy, Villigen, Switzerland; 2ETH Zürich, Department of Physics, Zürich, Switzerland; 3Paul Scherrer Insitute, Center for Proton Therapy, Villigen, Switzerland; 4GROW School for Oncology, Maastricht University Medical Centre+, Department of Radiation Oncology (Maastro), Maastricht, The Netherlands; 5University Hospital of Zurich, Department of Radiation Oncology, Zürich, Switzerland; 6Inselspital, Bern University Hospital, University of Bern, Department of Radiation Oncology, Bern, Switzerland
Show Affiliations
Hide Affiliations
Purpose or Objective
Tumour hypoxia causes radio resistance therefore, boosting the dose to hypoxic regions in the tumour can help improve the tumour control. We use baseline and in-treatment HX4 PET images to prepare dose escalated proton plans and compare them to the delivered photon plans in patients with head and neck cancer. We design a patient specific workflow for estimating the dose escalation necessary and use it to assess the potential of personalized and time adapted proton plans.
Material and Methods
HX4-PET images are used to delineate a hypoxic volume within the clinical target volume (CTV) with a tumour-to-muscle ratio > 1.4. A non-linear conversion model is used to obtain voxelised partial oxygen pressure (pO2), which along with variable linear energy transfer (LETd), is used for the proton-based oxygen enhancement ratio (OER) calculation. The patient specific dose escalation factor to the HTV (Hypoxic Target Volume) is defined using the calculated OER normalised to normoxic OER of the patient population. We investigate a split-treatment approach for varying hypoxic tumour sub-volumes at different points of time during treatment; at baseline and after 9-11 fractions of radiotherapy for a cohort of seven HNSCC (Head and Neck Squamous Cell Carcinoma) patients (fig 1).This split-treatment approach is compared against three plans; conventional IMRT/IMPT plans and hypoxia-adapted proton plan. Dosimetric differences within the plans were translated into predicting clinical outcomes with the help of tumour control probability (TCP) and normal tissue complication probability (NTCP) models.
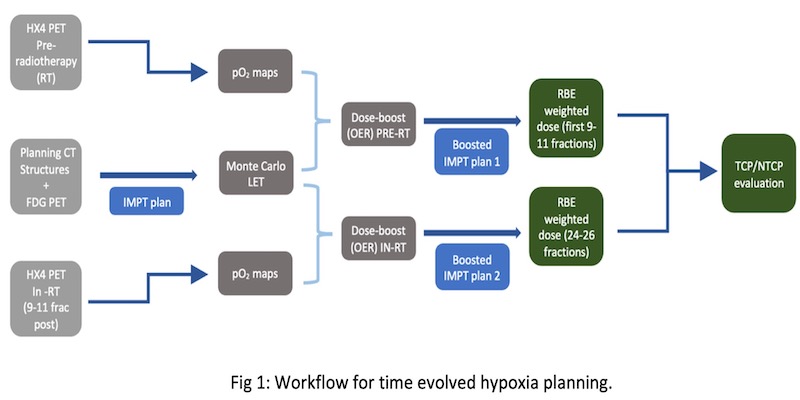
Results
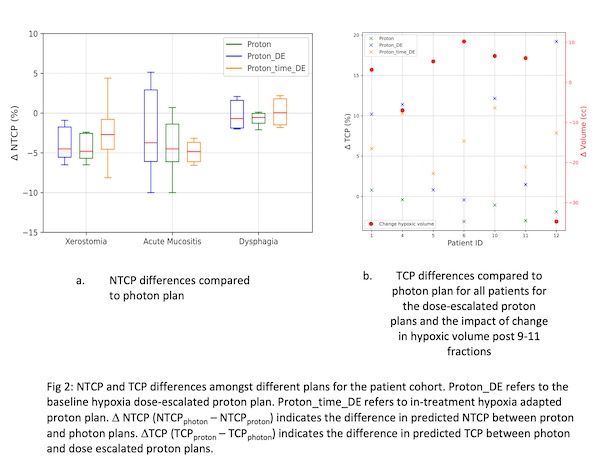
We observe a median increase of 60% in hypoxic volume after 9-11 fractions of radiotherapy along with spatial changes. However, the amount of hypoxia decreases indicating a median lower dose escalation factor of 9% as compared to 15% for overcoming the baseline hypoxia. We predict an improved median tumour control probability of 10% with the dose escalated plan and 7% with the split-treatment proton dose escalation compared to conventional proton plans. TCP is a function of the change in hypoxic volume with time as depicted in fig 2b. Larger hypoxic volume changes and a larger dose boost leads to an increased TCP. Median NTCP's over all patients were reduced for the three toxicities (xerostomia, dysphagia and acute mucositis) for all proton plans compared to the IMRT plans (deltaNTCP's < 0 in fig 2a). Furthermore, dose-escalated proton plans don’t lead to an increase in OAR dose as compared to conventional proton plans except in the cases where the organ at risk (e.g., superior pharyngeal constrictor muscles) lies within the planning volume which leads to an increase NTCP for dysphagia (fig 2a).
Conclusion
An evolving hypoxia based adaptive planning was studied. A workflow to consider varying hypoxia during the treatment has been designed. In conclusion, we observe better tumour control with a similar dose to the OARs with an adaptive planning approach compared to conventional proton plans.