A randomized trial of reduced elective neck dose in head & neck cancer: acute toxicity (NCT02442375)
Maurice Cox,
The Netherlands
OC-0829
Abstract
A randomized trial of reduced elective neck dose in head & neck cancer: acute toxicity (NCT02442375)
Authors: Maurice Cox1, Sven van den Bosch1, Patricia Doornaert2, Frank Hoebers3, Bas Kreike4, Marije Vergeer5, Ellen Zwijnenburg1, Tim Dijkema1, Johannes Kaanders1
1Radboud University Medical Center, Radiation Oncology, Nijmegen, The Netherlands; 2University Medical Center Utrecht, Radiation Oncology, Utrecht, The Netherlands; 3Maastro, Radiation Oncology, Maastricht, The Netherlands; 4Radiotherapiegroep, Radiation Oncology, Arnhem, The Netherlands; 5Amsterdam UMC, Radiation Oncology, Amsterdam, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
The objective of the UPGRADE-RT trial was to evaluate the safety and clinical benefit of elective neck radiotherapy with de-escalated dose compared to standard dose elective neck radiotherapy in patients with head and neck cancer. Here we report the acute toxicity.
Material and Methods
The UPGRADE-RT trial is a multicenter, randomized controlled, phase III trial (NCT02442375). In total 300 patients treated with radiotherapy for newly diagnosed stage T2-4N0-2M0 squamous cell carcinoma of the oropharynx, hypopharynx or larynx were randomized in a 2:1 ratio (intervention:control). For all patients a [18]FDG PET-CT scan in radiation treatment position was acquired. All patients received radiotherapy using an IMRT or VMAT technique with simultaneously integrated boost. An accelerated fractionation schedule was used, delivering 34 fractions in 5.5 weeks. Dose prescribed to the primary tumor CTV was 68 Gy. In the intervention arm, patients received elective neck irradiation with de-escalated dose (43 Gy). In the control arm, patients received elective neck irradiation with standard dose (50 Gy). The primary endpoint was ‘normalcy of diet’ at 1 year after treatment (clinical benefit). The secondary endpoint was the actuarial rate of recurrence in electively irradiated lymph nodes at 2 years after treatment (safety). Here we present the acute toxicity during treatment until 90 days after start of treatment. Radiation induced dermatitis, mucositis, pharyngeal dysphagia and tube feeding were scored during radiotherapy until complete recovery, according to the common toxicity criteria version 2.0. Acute toxicity was compared between groups using the chi-square test.
Results
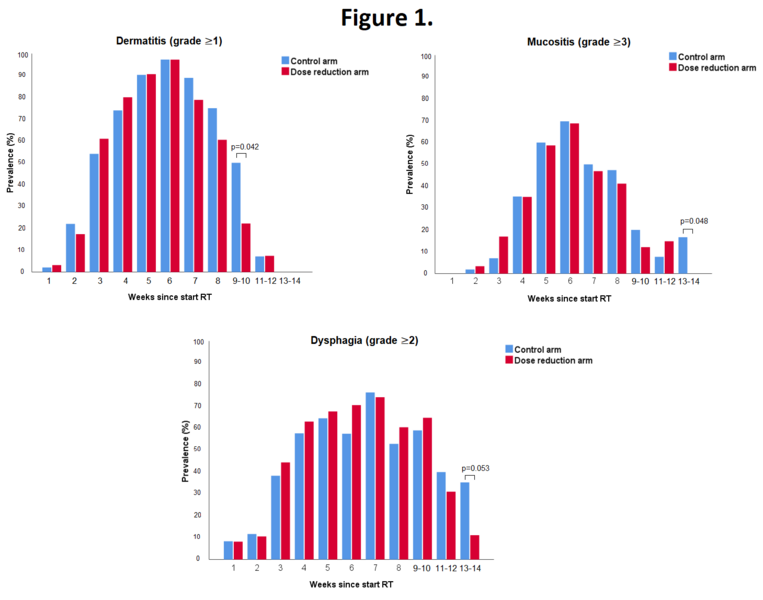
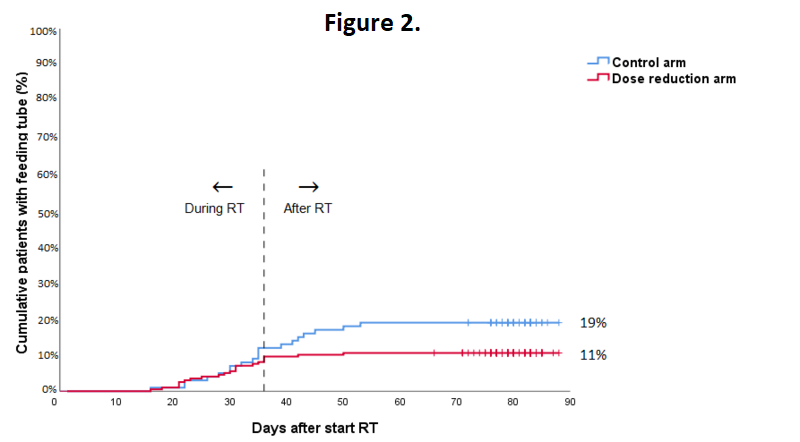
In total 295 patients were evaluable. 5 patients were excluded for reasons of patient refusal (n=2), prior oncologic surgery of the neck (n=1), concomitant chemotherapy (n=1) or total laryngectomy (n=1). The highest acute toxicity scores during treatment were not statistically significant different between treatment groups. Grade 3 to 4 acute adverse events occurred in 70% of all patients, with mucositis being most frequently reported. The mean number of grade 3-4 adverse events per patient was similar in the control arm and the dose reduction arm (1.0 and 0.9, respectively, p=0.337). In the dose reduction arm, an earlier recovery of grade ≥1 dermatitis (week 9-10; 22% vs. 50%; p=0.042), grade ≥3 mucositis (week 13-14; 0% vs. 17%; p=0.048) and grade ≥2 dysphagia (week 13-14; 11% vs. 35%; p=0.053) was observed (Figure 1). Tube feeding was required in 11% vs. 19% of patients in the dose reduction arm and control arm, respectively (p=0.045; Figure 2). The median duration of tube feeding was 36 days (interquartile range (IQR): 23-55) in the dose reduction arm and 34 days (IQR 22-39) in the control arm (p=0.307).
Conclusion
Radiation dose de-escalation to the elective neck in patients with head and neck cancer, resulted in a significantly lower rate of patients requiring tube feeding and a faster recovery of mucositis and dysphagia.