Impact of radiation to host immune system in patients treated for locally advanced NSCLC
Corentin Pasquier,
France
OC-0261
Abstract
Impact of radiation to host immune system in patients treated for locally advanced NSCLC
Authors: Corentin Pasquier1, Léonor Chaltiel2, Carole Massabeau1, Audrey Rabeau3, Louisiane Lebas4, Jean-Sébastien Texier5, Nathalie Caunes-Hilary6, Elizabeth Cohen-Jonathan Moyal1, Julien Mazières3, Jonathan Khalifa1
1Institut Universitaire du Cancer Toulouse - Oncopole, Department of Radiotherapy, Toulouse, France; 2Institut Universitaire du Cancer Toulouse - Oncopole, Department of Biostatistics, Toulouse, France; 3Centre Hospitalier Universitaire de Toulouse, Hôpital Larrey, Thoracic Oncology Department, Toulouse, France; 4Centre Hospitalier Intercommunal des Vallées de l'Ariège (CHIVA), Department of Pneumology, St-Jean de Verges, France; 5Institut Universitaire du Cancer Toulouse - Oncopole, Department of Nuclear Medicine, Toulouse, France; 6Institut Universitaire du Cancer Toulouse - Oncopole, Department of medical oncology, Toulouse, France
Show Affiliations
Hide Affiliations
Purpose or Objective
The optimal modalities of radiotherapy when combining concurrent chemoradiation (CCRT) and immunotherapy (IO) for unresectable locally advanced non-small cell lung cancer (NSCLC) remain to be determined. The aim of this study was to investigate the impact of radiation to different immune structures and immune cells upon clinical outcomes in patients treated by CCRT followed by durvalumab.
Material and Methods
Patients with locally advanced NSCLC treated with CCRT and consolidation IO were analysed. Dosimetric data such as dose to non-involved tumor draining lymph nodes (NITDLN), vertebrae (T1-T12), spleen or estimated radiation dose to immune cells (EDRIC) were collected. Patients were divided into two groups according to the inclusion (NILN-R+) or not (NILN-R-) of at least one NITDLN in the clinical target volume (CTV). Complete blood counts were assessed before CCRT, at the end of CCRT and at the initiation of consolidative IO. Progression free survival (PFS) and overall survival (OS) were estimated by the Kaplan-Meier method with 95% confidence intervals (CI). Univariable and multivariable analysis were performed using the Logrank test and the Cox proportional hazards model.
Results
Fifty patients were included with a median follow-up of 23.2 months (95% CI 18.3-35.2).
Median PFS was 31.4 months (95% CI 14.0 – not reached). 2-year overall survival (OS) was 66.2% (95% CI 46,5 – 80,1).
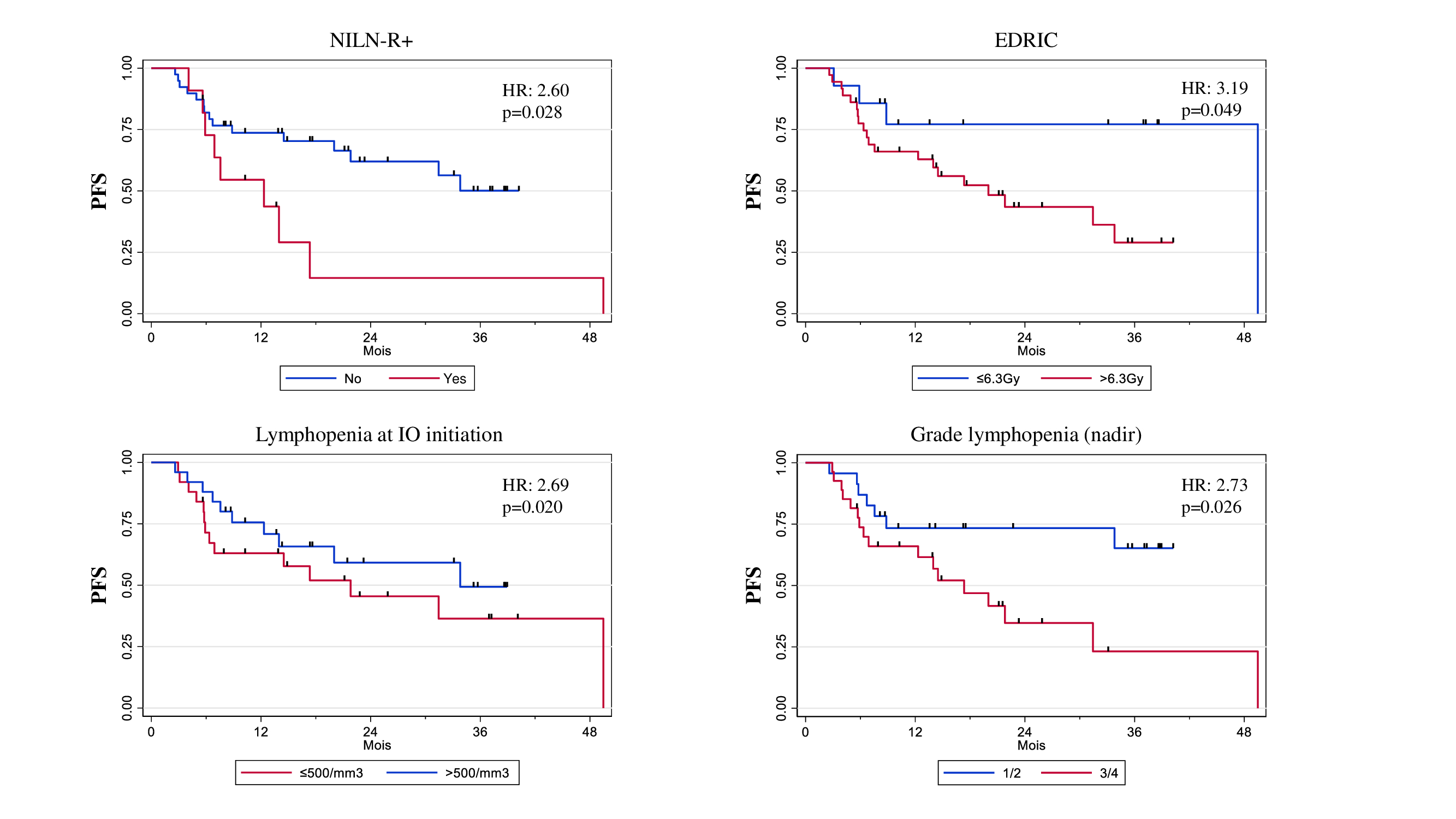
In univariable analysis, NILN-R+ (HR: 2.60, 95%CI 1.08-6.27; p=0.028), EDRIC > 6.3 Gy (HR: 3.19, 95%CI 0.94-10.82; p=0.049) and lymphopenia ≤500/mm3 at IO initiation (HR: 2.69, 95%CI 1.12-6.46; p=0.021) were correlated with PFS and lymphopenia ≤ 500/mm3 at IO initiation was also associated with poor OS (HR: 3.46, 95%CI 1.10-10.87;p=0.024).
In multivariable analysis, NILN-R+ was the strongest factor associated with PFS (HR: 3.15, 95%CI 1.23-8.10; p=0.017).
Conclusion
The inclusion of at least one NITDLN station within the CTV (NILN-R+) was an independent factor for poor PFS in the context of CCRT and durvalumab consolidation for locally advanced NSCLC. Therefore, the optimal sparing of immune structures might be of interest in order to achieve a better synergy between radiotherapy and immunotherapy in this indication.