The longitudinal effect of neoadjuvant radiotherapy on lymphocytes in primary breast cancer
Miki Yoneyama,
United Kingdom
OC-0262
Abstract
The longitudinal effect of neoadjuvant radiotherapy on lymphocytes in primary breast cancer
Authors: Miki Yoneyama1, Konstantinos Zormpas-Petridis1, Ruth Robinson1,2, Faranak Sobhani3, Ioannis Roxanis4, Elena Provenzano5, Harriet Steel1, Sara Lightowlers6, Meena Murthy7, Catherine Philpot6, Simon P. Castillo3, Tom Lund8, Erik Wennerberg1, Alan Melcher1, Charlotte Coles6, Yinyin Yuan3, Navita Somaiah1,2
1The Institute of Cancer Research, Division of Radiotherapy and Imaging, London, United Kingdom; 2The Royal Marsden NHS Foundation Trust, Breast Unit, London, United Kingdom; 3The Institute of Cancer Research, Division of Molecular Pathology, London, United Kingdom; 4The Institute of Cancer Research, The Breast Cancer Now Toby Robins Research Centre, London, United Kingdom; 5Cambridge University Hospitals NHS Foundation Trust, Pathology, Cambridge, United Kingdom; 6University of Cambridge, Department of Oncology, Cambridge, United Kingdom; 7University of Cambridge, Department of Medicine, Cambridge, United Kingdom; 8The Institute of Cancer Research, Integrated Pathology Unit, London, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
Despite the growing interest in combining radiotherapy (RT) with immune checkpoint blockers (ICB), there is a lack of understanding on the effect of RT itself on the human tumour-immune microenvironment (TIME) and thus the anti-tumour immune response. The PRADA (NCT02771938) and Neo-RT (NCT03818100) trials, which tested feasibility and safety of neoadjuvant RT (NRT) in breast cancer patients, represent a unique opportunity to study novel in vivo longitudinal radiobiological data from the treatment-naïve TIME. We characterised NRT-induced changes to stromal tumour-infiltrating lymphocytes (sTILs) and peripheral blood lymphocytes in these cohorts.
Material and Methods
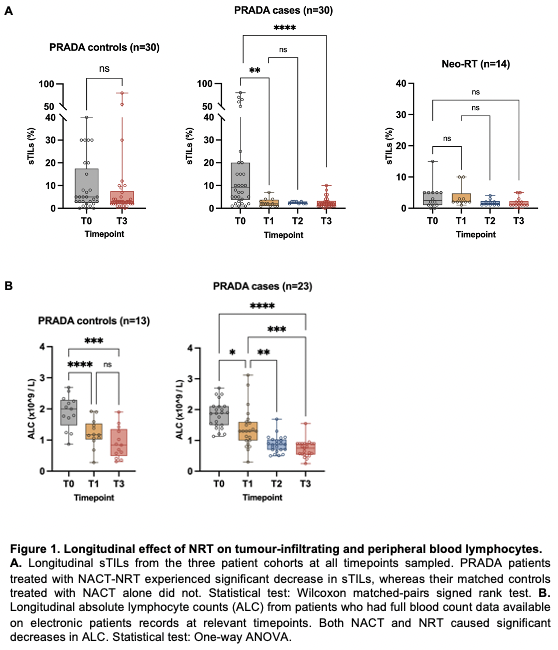
PRADA cases (n=30) received pre-operative locoregional RT (40Gy/15F) following neoadjuvant chemotherapy (NACT). Contemporaneous case-matched controls for PRADA (n=30) received NACT alone. Neo-RT (n=13) received pre-operative locoregional RT (40Gy/15F, with simultaneous integrated boost of 48Gy/15F to primary tumour where possible) followed by 20 weeks of pre-operative endocrine therapy. FFPE samples were obtained from primary tumour at diagnosis (T0), after one fraction of NRT (T1), after final fraction of NRT (T2), and at surgery (T3). H&E-stained sections were manually scored by a breast pathologist blinded to clinical context, following the International TILs Working Group guidelines. Digitised H&E sections were also scored using artificial intelligence (AI) methods for comparison. Absolute lymphocyte count (ALC) from full blood count data at relevant timepoints was evaluated for a subset of patients from PRADA.
Results
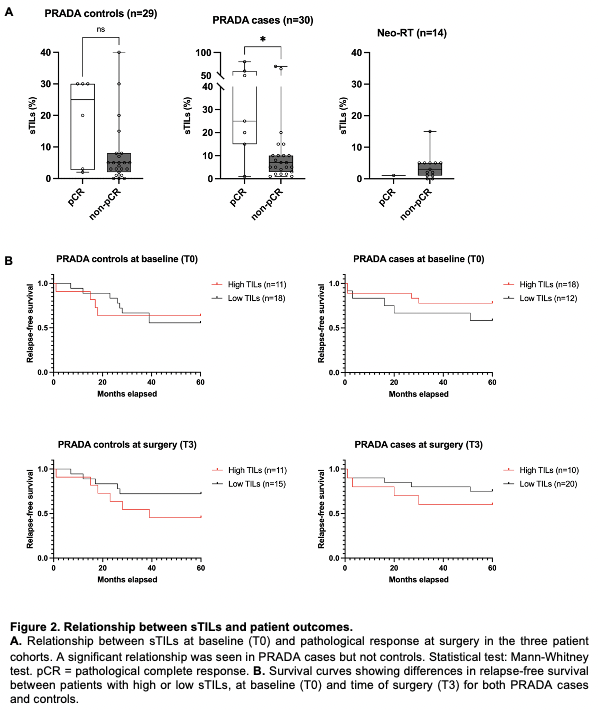
PRADA cases experienced a significant and uniform decrease in sTILs between T0 and subsequent timepoints, with no recovery by T3 (Fig.1A). This was not seen in PRADA controls following NACT alone. sTILs also showed a trend towards decreasing infiltration in Neo-RT. Both NACT and NRT contributed to significant decreases in ALC (Fig.1B). High sTILs at T0 was associated with pathological complete response (pCR) in PRADA cases only, but a relationship between sTILs at T3 and relapse-free survival was seen in both PRADA cases and controls (Fig.2A,B). AI-TILs analysis using a novel approach combining cell classification with the SuperHistopath pipeline showed superior concordance with manual scores when compared with other AI methods. A model using combined manual-AI scores had greater AUC for prediction of pCR compared to models using either parameter alone.
Conclusion
Our data suggest a locally lymphodepletive effect of NRT on breast TIME with NRT dose-volumes and sequencing schedules used in these trials. However, the correlations between outcome and sTILs at both baseline and time of surgery in patients receiving NRT suggest a complex underlying relationship between RT and the immune response. Ongoing work on these samples including multiplex immunofluorescence and TCR-seq will elucidate this further, and ultimately inform the rational design of future RT-ICB combination trials.