Single fraction SBRT vs 3D-CRT for painful bone metastases: a single-blind phase 3 RCT (NCT03831243)
OC-0762
Abstract
Single fraction SBRT vs 3D-CRT for painful bone metastases: a single-blind phase 3 RCT (NCT03831243)
Authors: Carole Mercier1,2, Charlotte Billiet1,2, Geert De Kerf1, Katrien Vandecasteele3,4, Piet Ost1,4, Ines Joye1,2, Peter Vermeulen5,2, Luc Dirix6, Yolande Lievens3,4, Dirk Verellen1,2, Piet Dirix1,2
1Iridium Netwerk, Radiation Oncology, Antwerp, Belgium; 2University Antwerp, Integrated Personalised and Precision Oncology Netwerk, Antwerp, Belgium; 3University Hospital Ghent, Radiation Oncology, Gent, Belgium; 4Ghent University, Department of Human Structure and Repair, Gent, Belgium; 5GZA ziekenhuizen, Translational Cancer Research unit, Wilrijk, Belgium; 6GZA Ziekenhuizen, Medical Oncology, Wilrijk, Belgium
Show Affiliations
Hide Affiliations
Purpose or Objective
To test whether high-dose single fraction SBRT of 20 Gy to painful bone metastases is superior to standard 8 Gy 3D-conformal radiotherapy (3D-CRT) in achieving complete pain responses (CR).
Material and Methods
In this single-blind, multicentre, randomised phase 3 trial, patients with up to 3 painful bone metastases were randomly assigned (1:1) to either a single SBRT fraction of 20 Gy or a single 3D-CRT treatment with 8 Gy. Inclusion criteria consisted of uncomplicated painful (worst pain score of ≥2 on a 0-10 pain scale) bone metastases arising from a solid tumour. The primary endpoint was the proportion of patients with a CR for pain at 1-month after RT, analysed as per intention-to-treat principle. Secondary endpoints included pain flare, re-irradiation need, toxicity (CTCAE version 5.0), quality of life (QoL). The study had 80% power to detect an absolute 25% improvement in 1-month CR rate from 25% to 50% in favour of SBRT. At the time of the current analysis, all patients completed 3 months of follow-up. The trial was registered on ClinicalTrials.gov (NCT03831243) and received funding from Kom Op Tegen Kanker.
Results
Between April 2019 and October 2022, 126 patients were randomised. Baseline characteristics were well-balanced between both arms (Table 1). In the SBRT group, three patients were unable to undergo SBRT.
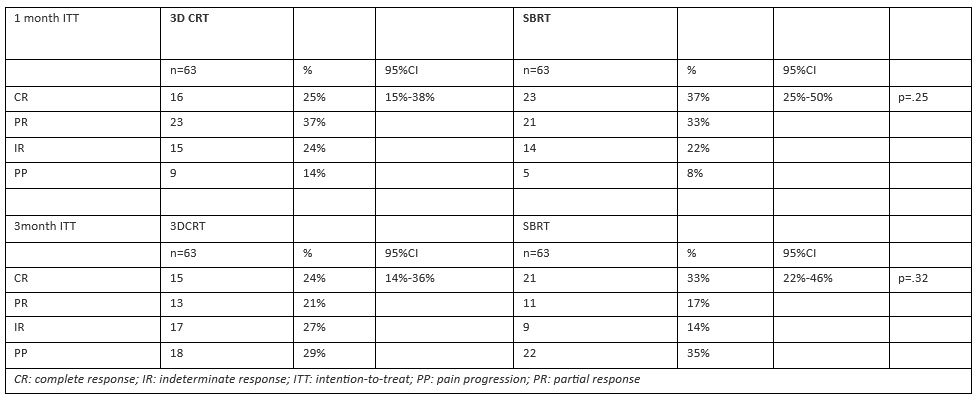
At one month, 16/63 (25%) patients achieved CR after 3D-CRT vs. 23/63 (37%) after SBRT (p=.25, Table 2). At 3 months, 15/63 (24%) patients achieved CR after 3D-CRT vs. 21/63 (33%) after SBRT (p=.32, Table 2). A per-protocol analysis of patients still evaluable after 3 months (87/126, 69%) showed a significantly higher CR after SBRT (21/39, 54%) compared to 3D-CRT (15/48, 31%, p=.048).
Twenty-seven percent vs. 18% of patients experienced a post-radiation pain flare (p=.27). Re-irradiation of the painful lesion was indicated for 11% vs. 2% of patients (p=.06) in the 3D-CRT and SBRT arm, respectively.
Grade 2-3 toxicity was observed in 14% and 15% in the 3D-CRT and SBRT arms, respectively, and no Grade ≥4 events occurred. QoL outcomes did not differ between the groups.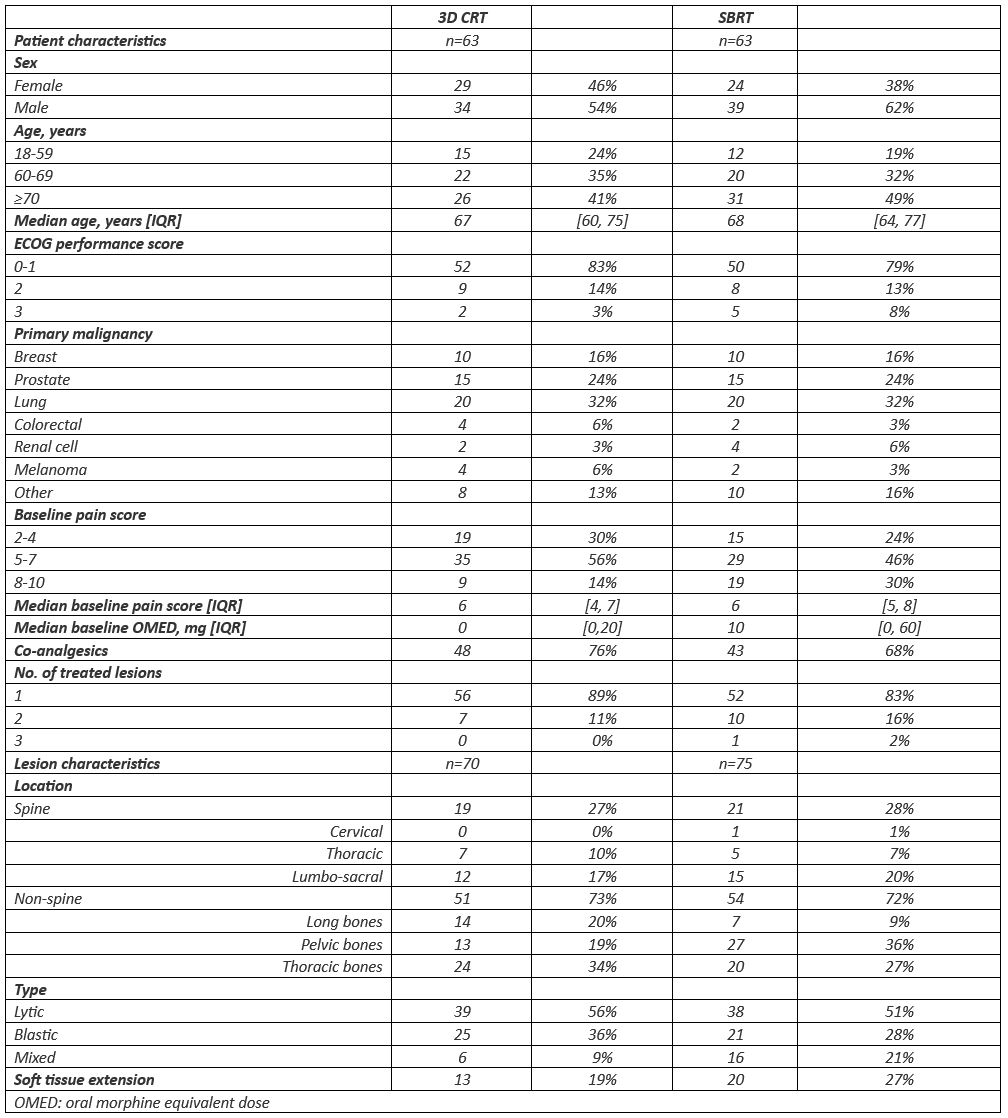
Conclusion
This is the first randomised controlled trial comparing SBRT to 3D-CRT for pain relief in both spine and non-spine bone metastases where patients remained blinded to their treatment arm.
SBRT was safe and did not result in any excess toxicity. Dose-escalation improved complete pain response rate 1 month after radiotherapy, although less than anticipated and not statistically significant. However, for patients with an expected survival of at least 3 months, there might be a clinically relevant benefit.