Phase 2 trial of FOLFIRINOX followed by chemoradiation in locally advanced pancreatic cancer
Gian Marco Petrianni ,
Italy
PD-0892
Abstract
Phase 2 trial of FOLFIRINOX followed by chemoradiation in locally advanced pancreatic cancer
Authors: Gabriele D'Ercole1, Michele Fiore2, Gian Marco Petrianni3, Pasquale Trecca3, Martina Benincasa1, Edy Ippolito2, Damiano Caputo4, Giuseppe Tonini5, Roberto Coppola4, Sara Ramella2
1Università Campus Bio-Medico di Roma, Research Unit of Radiation Oncology, Rome, Italy; 2Università Campus Bio-Medico di Roma, Fondazione Policlinico Universitario Campus Bio-Medico, Research Unit of Radiation Oncology, Rome, Italy; 3Fondazione Policlinico Universitario Campus Bio-Medico, Operative Research Unit of Radiation Oncology, Rome, Italy; 4Università Campus Bio-Medico di Roma, Fondazione Policlinico Universitario Campus Bio-Medico, Research Unit of General Surgery, Rome, Italy; 5Università Campus Bio-Medico di Roma, Fondazione Policlinico Universitario Campus Bio-Medico, Research Unit of Medical Oncology, Rome, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
To evaluate the margin-negative (R0) resection rate in borderline resectable and locally advanced unresectable pancreatic ductal adenocarcinoma (PDAC) after induction FOLFIRINOX followed by chemoradiotherapy (CRT).
Material and Methods
We evaluated patients with locally advanced PDAC for enrolment in a single-arm, phase 2 clinical trial. Eligible patients underwent CT scan, 18FDG PET-CT scan and a staging laparoscopy to detect occult metastasis before the treatment. Patients received FOLFIRINOX regimen for 4 cycles. For patients without disease progression as detected through restaging exams, chemotherapy was followed by long-course CRT, which consisted of Volumetric Modulated Arc Therapy (VMAT) and concurrent gemcitabine at the dose of 600 mg/mq weekly. Four weeks after the completion of CRT, CT scan and PET-CT scan were performed. Surgery was considered in patients whose tumours were technically resectable. The primary objective was R0 resection rate with an alternate hypothesis of 55%. Secondary objectives included progression-free survival (PFS), overall survival (OS), local progression-free survival (LPFS), metastases free-survival (MFS), and safety. The occurrence and nature of any adverse events were recorded in accordance with the National Cancer Institute Common Toxicity Criteria (version 4.0) scale. The trial registration number is NCT05399394.
Results
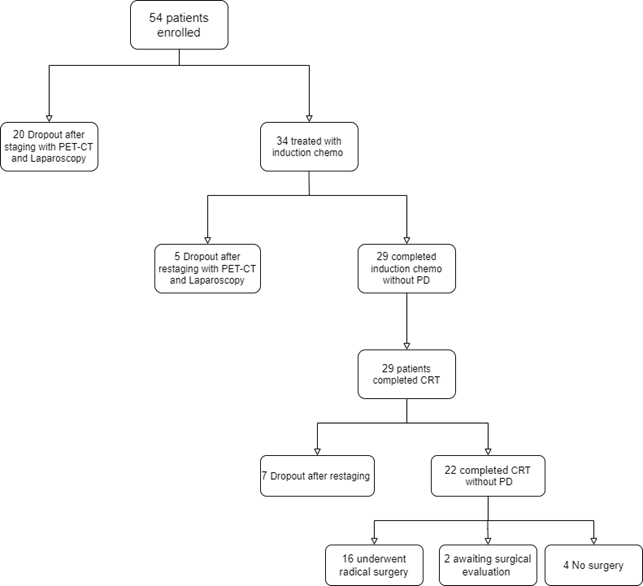
Of the 54 eligible patients (31 men, 23 women), median age was 63 years (range, 48-75 years). Twenty patients (37%) were excluded because of the evidence of metastatic disease, and thus thirty-four patients were consequently enrolled. Of these, fourteen patients (41%) had locally advanced unresectable tumours, twenty patients (59%) had borderline resectable disease. Five patients (14.7%) had a progression of disease after induction chemotherapy. Twenty-nine patients received concomitant CRT (Figure). All patients completed the combined treatment. Eighteen patients were evaluated with surgical exploration, and sixteen patients (55%) underwent surgical radical resection. Two patients are awaiting surgical evaluation. R0 resection was achieved in all 16 of the 29 eligible patients (55%). The median follow-up was 17 months (range, 6 to 63 months). Median OS and median PFS in patients who have completed CRT were 17 months (95% CI, 13 to 22) and 12 months (95% CI, 9.8 to 16.1), respectively. One-year OS, one-year PFS, one-year LPFS and one-year MFS were 88%, 53%, 80% and 65%, respectively. Patients who underwent resection had a significant longer median OS compared with non resected patients (22.4 months vs 13.2 months, p=0.03). Median PFS for resected patients was 14.5 months compared with 8.7 months for non resected patients (p<0.001). Treatment-related grade 3 to 4 toxicities included neutropenia (20%), nausea and vomiting (7%), diarrhea (4%).
Conclusion
FOLFIRINOX followed by long-course CRT in borderline resectable and locally advanced unresectable PDAC results in high rates of R0 resection and prolonged median PFS and median OS.