Patient-reported outcomes after rectal cancer treatment on a 1.5T MR-Linac within the MOMENTUM study
Lois Daamen,
The Netherlands
PD-0885
Abstract
Patient-reported outcomes after rectal cancer treatment on a 1.5T MR-Linac within the MOMENTUM study
Authors: Lois Daamen1, Jasmijn Westerhoff1, Alice Couwenberg2, Petra Braam3, Heidi Rütten3, John Christodouleas4, William Hall5, Helena Verkooijen1, Martijn Intven6
1University Medical Center Utrecht, Division of Imaging and Oncology, Utrecht, The Netherlands; 2The Netherlands Cancer Institute, Radiation Oncology, Amsterdam, The Netherlands; 3Radboud University Medical Center, Radiation Oncology, Nijmegen, The Netherlands; 4University of Pennsylvania, Radiation Oncology, Philadelphia, USA; 5Medical College of Wisconsin, Radiation Oncology, Milwaukee, USA; 6University Medical Center Utrecht, Radiation Oncology, Utrecht, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
With the 1.5 Tesla (T) Magnetic Resonance Linear Accelerator (MR-Linac), planning target volume (PTV) margins for the treatment of rectal cancer can be reduced by 1/3 compared to conventional radiotherapy techniques. This allows better sparing of surrounding tissues, potentially resulting in less toxicity and greater patient comfort. Patient-reported outcomes (PROs) after rectal cancer treatment on a 1.5T MR-Linac have not yet been reported. Through international collaboration, the ‘Multi-OutcoMe EvaluatioN of radiation Therapy Using the MR-Linac (MOMENTUM)’ registry provides the unique opportunity to study these outcomes in a relatively large patient cohort. The aim of this study is to assess PROs of rectal cancer patients treated on a 1.5T MR-Linac within MOMENTUM.
Material and Methods
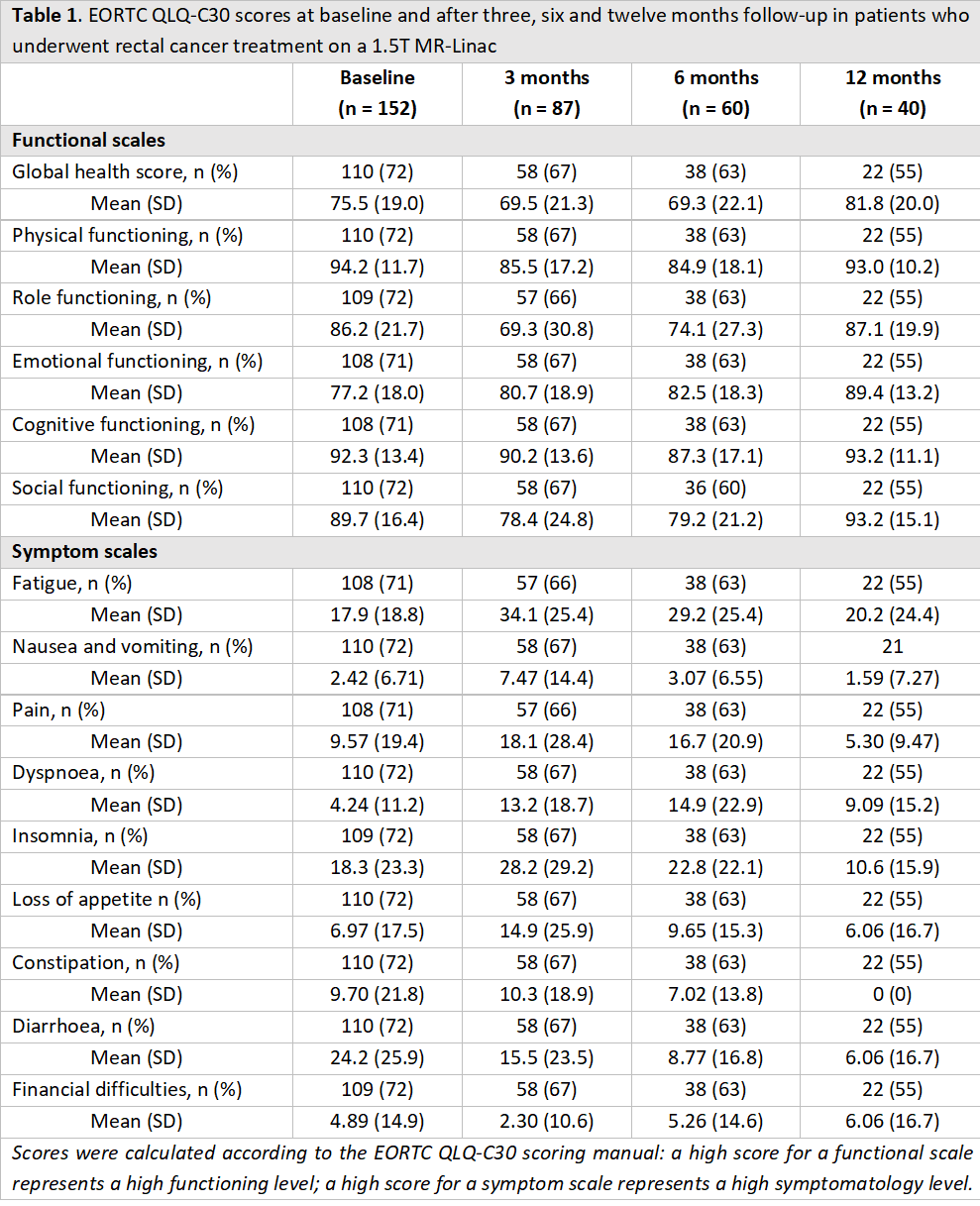
An international, prospective, observational cohort study was performed, including all patients with rectal cancer who were treated with 25 Gy in five fractions on an MR-Linac in three institutions located in the Netherlands (2019-2022). Patient-reported outcomes were measured using the general EORTC QLQ-C30 and colorectal cancer-specific EORTC QLQ-CR29 questionnaires. Scores were calculated according to the EORTC QLQ-C30 scoring manual. A high score for a functional scale represents a high functioning level, while a high score for a symptom scale represents a high symptomatology level. Outcomes were measured at regular time points (i.e., at baseline and after three, six and twelve months follow-up). Patients who underwent tumor resection were censored at the date of surgery.
Results
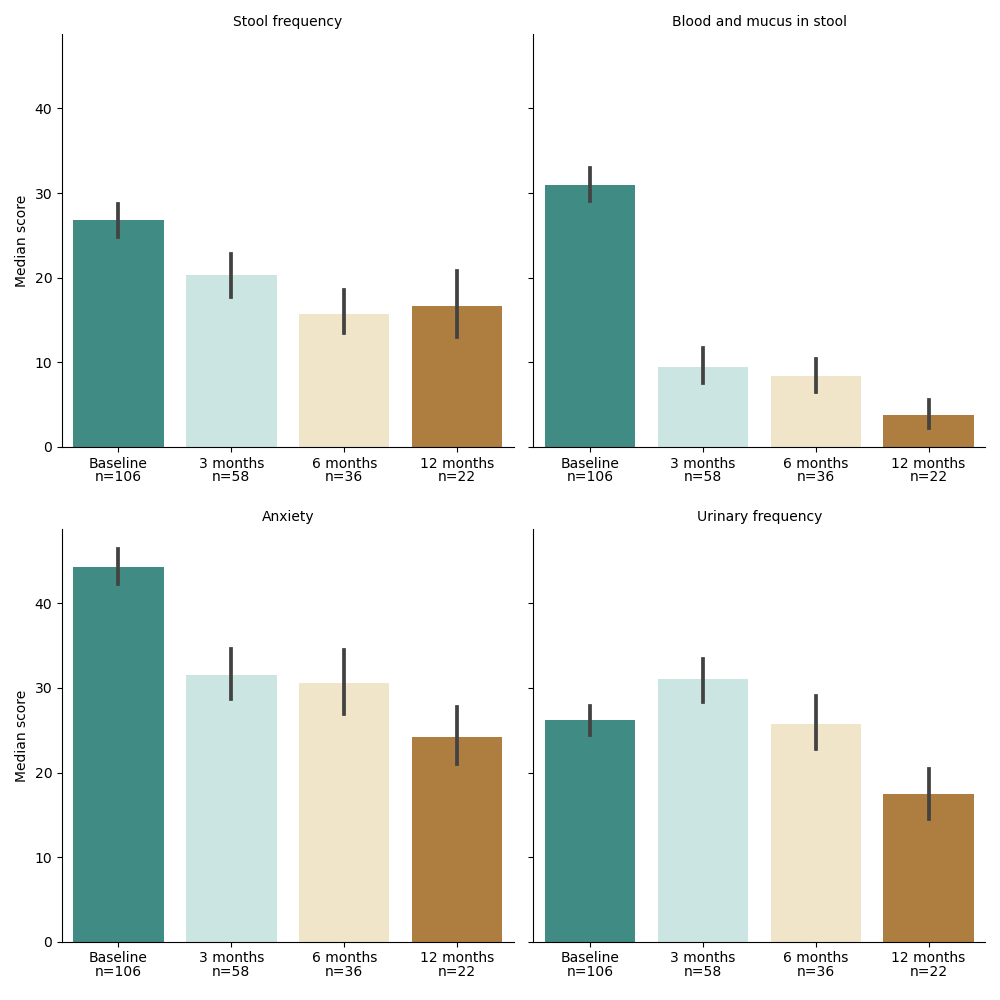
In total, 152 patients were included, with a median follow-up of 17 (interquartile range [IQR] 11-27) months. Of these, six patients (4%) had American Joint Committee on Cancer (AJCC) stage I disease, 11 patients (7%) stage II, 85 patients (56%) stage III, and 12 patients (8%) stage IV. A total of 87 patients (57%) reached three months follow-up without surgery, 60 patients (39%) six months, and 40 patients (26%) twelve months. At six months follow-up, 25/132 patients (19%) had received additional chemotherapy. The median global health score was 83 (interquartile range [IQR] 67-83) at baseline, 75 (IQR 58-83) at three months, 75 (IQR 58-83) at six months and 83 (IQR 83-100) at twelve months. In most QLQ-C30 domains, functional and symptom scores deteriorated during the first six months after treatment but returned to or exceeded baseline scores at 12 months (Table 1). Overall, treatment resulted in improved QLQ-CR29 scores after 12 months follow-up. Most frequently reported symptoms were blood and mucus in stool, urinary frequency, stool frequency and anxiety (Figure 1).
Conclusion
This study presents patient-reported outcomes in the currently largest cohort of patients with rectal cancer who received treatment on a 1.5T MR-Linac. Overall, treatment resulted in improved symptom management, and stabilized or improved quality of life outcomes after twelve months of follow-up.