Dosimetric analysis of bronchial tree to assess toxicity-risk in SBRT of ultracentral lung tumors
Maiwand Ahmadsei,
Switzerland
PD-0154
Abstract
Dosimetric analysis of bronchial tree to assess toxicity-risk in SBRT of ultracentral lung tumors
Authors: Maiwand Ahmadsei1, Vinojaa Jegarajah1, Riccardo Dal Bello1, Sebastian M. Christ1, Michael M. Mayinger1, Luisa Sabrina Stark1, Jonas Willmann1, Ivan R. Vogelius2, Panagiotis Balermpas1, Nicolaus Andratschke1, Stephanie Tanadini-Lang1, Matthias Guckenberger1
1University Hospital Zurich, Department of Radiation Oncology, Zurich, Switzerland; 2Rigshospitalet, University of Copenhagen, Department of Oncology, Copenhagen, Denmark
Show Affiliations
Hide Affiliations
Purpose or Objective
Stereotactic body radiotherapy (SBRT) is a guideline-recommended treatment option for patients with medically inoperable early stage lung cancer and pulmonary oligometastases. In case of ultra-central tumor location - defined as tumor contact (PTV) with proximal bronchial tree (PBT), trachea or esophagus - SBRT is associated with an increased risk of severe toxicity. Therefore, the role of SBRT for ultra-central lung tumors remains controversial. The aim of this study was to perform a detailed dosimetric analysis of PBT subsegments to evaluate safety of SBRT in ultra-central lung tumors.
Material and Methods
Fifty-seven ultra-central lung tumor patients treated with SBRT at our institution from 2014 to 2021 were included. Ultra-central lung tumors were defined as tumor (PTV) abutting or involving trachea, PBT or esophagus. Descriptive analysis, Cox regression, Kaplan-Meier method and log-rank test were employed to analyze overall survival (OS), local control (LC) and progression-free survival (PFS). Bayesian inference was employed to build a dose-response model for toxicity. While the prior was based on meta-analysis of previous literature, the likelihood was computed based on a binomial distribution taking into account the number of observed patients without toxicity.
Results
Twenty-seven (47.4%) of the irradiated lesions were primary lung tumors and 30 (52.6%) metastases.The most common primary tumor was non-small cell lung cancer (NSCLC) with 37 (64.9%) cases, of which 12 patients had primary non-metastatic NSCLC, and 25 patients had locally recurrent or oligometastatic NSCLC. Colorectal cancer, small-cell lung cancer, head-and-neck cancer, metastatic melanoma and sarcoma combined accounted for twenty oligometastatic patients (35.1%). Patients were treated with risk-adapted SBRT of median 45.0 Gy (30.0-60.0 Gy) respectively 55.2 Gy (33-88 Gy, EQD2, α/β = 10 Gy) in 8 or 10 fractions. The most commonly prescribed dose was 48 Gy in 8 fractions (n=30, 53.0%). The median prescription isodose to the PTV was 65.0% (65.0-85.0%). The median planning target volume was 30.0 cm3 (6.0-199.0 cm3). After a median follow-up of 2.2 years (0.6-9.3 y), the one-year and two-year LC rates were 85.2% and 77.1%, respectively. The median OS was 3.4 years (0.6-9.3 y), OS at 2 years was 71.3%. The median PFS was 1.0 year (0.2-5.7y). Grade ≥3 radiation pneumonitis was observed in two patients (3.5%), while no bronchial stenosis, hemorrhage or fistula was observed. The median D0.02cc to PBT was 84.4 Gy (EQD2, α/β = 3 Gy: 43.9-159.3 Gy). The dose-response model predicted a toxicity limited to 4.9% (0 - 11.4%) when delivering 100 Gy (EQD2, α/β = 3 Gy) to any location of the PBT (D0.2cc) (Figure 1b).
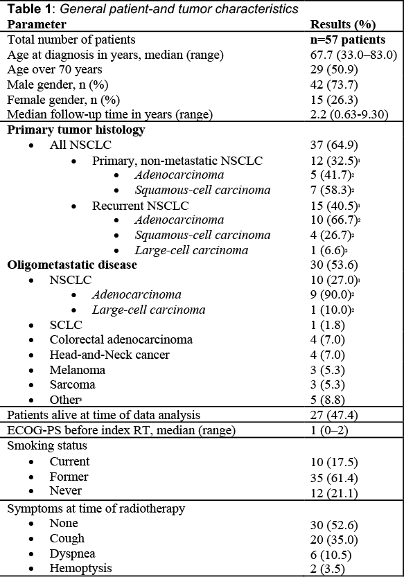
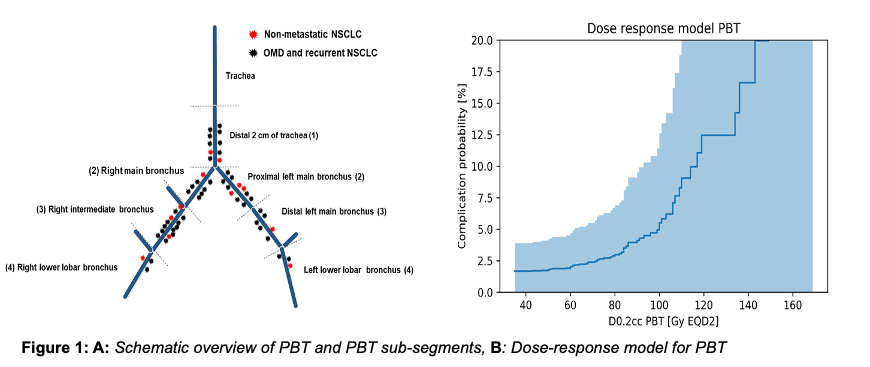
Conclusion
This study reports a detailed dosimetric analysis of PBT subsegments after SBRT for ultra-central lung tumors. A dose threshold of 100Gy EQD2 to the PBT and its sub-segments is expected to result in low rates of severe bronchial toxicity, while maintaining high rates of local tumor control.