The value of post Radiotherapy PSA dynamics for prostate cancer risk stratification models
Jane Shortall,
United Kingdom
PD-0164
Abstract
The value of post Radiotherapy PSA dynamics for prostate cancer risk stratification models
Authors: Jane Shortall1, Eliana Vasquez-Osorio1, Andrew Green2, David Wong3, Peter Hoskin1, Ananya Choudhury1, Marcel van Herk1, Alan McWilliam1
1The University of Manchester, Faculty of Biology, Medicine, Division of Cancer Sciences, Manchester, United Kingdom; 2European Bioinformatics Institute, European Molecular Biology Laboratory, Cambridge, United Kingdom; 3The University of Manchester, Division of Informatics, Imaging & Data Sciences, Manchester, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
One in three men with prostate cancer (PCa) experience biochemical recurrence (BCR), detected via rises in Prostate Specific Antigen (PSA). Although post-radiotherapy (RT) PSA characteristics may be associated with BCR, they are not currently included in prognostic models, missing an opportunity for ‘early’ intervention, e.g increased monitoring frequency.
We investigated whether adding short-term postRT PSA characteristics in risk stratification models improved prediction of future BCR.
Material and Methods
Patient data, including repeat postRT PSA measurements, were collected for 254 men treated with conformal hypo-fractionated RT at a single institution between 2005 and 2007 (mean follow-up 6.8years). This cohort only included patients with complete follow-up, no BCR within 3 years of RT, ≥2 PSA readings and recorded nadir for the same period.
PSA trajectories were modelled up to 3 years postRT using Gaussian Process (GP) regression (pythonv3.6.5,GPy). Models were sampled at regular intervals (0.1years) to predict time to nadir.
To characterise PSA dynamics, models were compared among all patients using the standard deviation of the Euclidian distance between trajectories. These were then clustered using hierarchical clustering.
Cox proportional-hazard models with and without postRT PSA information (first postRT PSA, predicted time to nadir, cluster assignment) were produced and the Akaike Information Criterion (AIC) used to assess model performance.
Results
77/253 men recurred after 3 years postRT (nadir+2ng/ml). PSA trajectories were grouped into 3 clusters (Fig1A-C) and Kaplan-Meier analysis showed a significant difference in future BCR between these clusters (Fig1D) (p<0.001).
GP models predicted nadir as or before nadir was recorded for 214/253 men (66/74 who recurred) (Fig1E), validating GP as an interpolation technique.
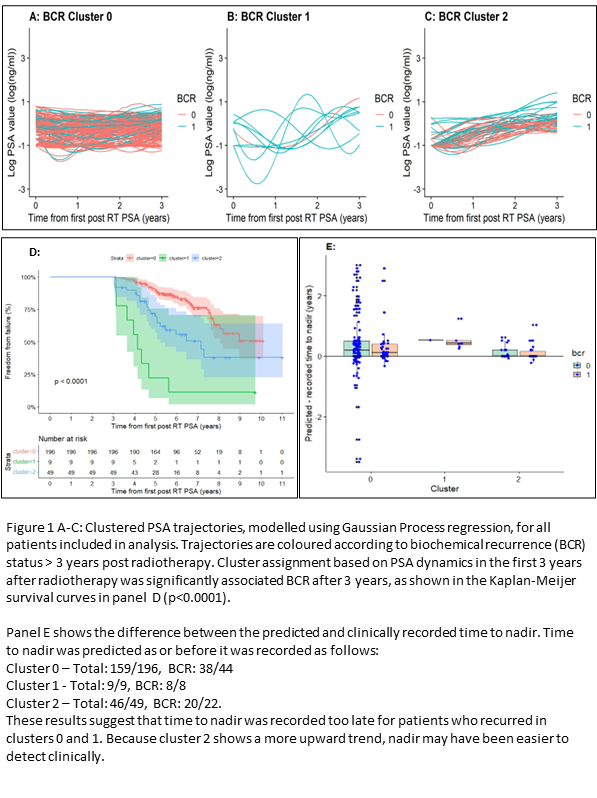
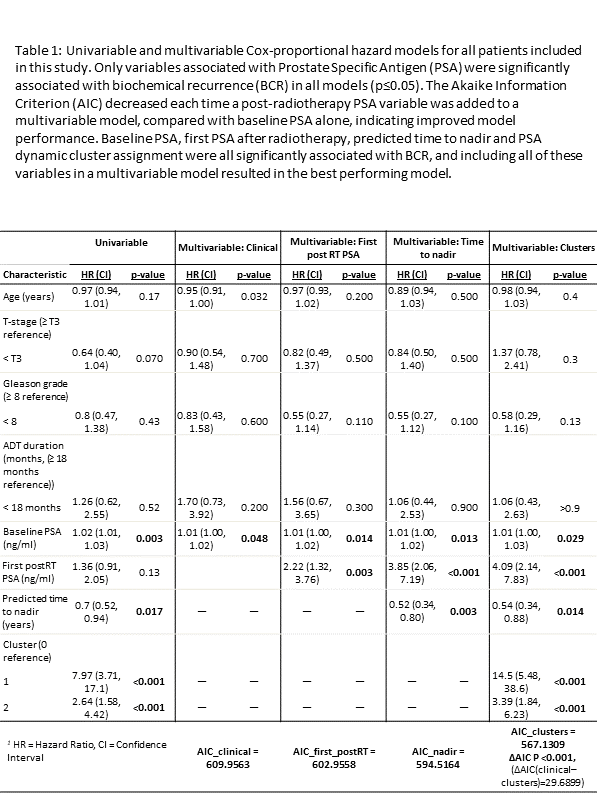
Table 1 shows statistical models. Univariable analyses showed that baseline PSA, predicted time to nadir and cluster assignment were significantly associated with BCR (p≤0.017). These variables remained significant in all multivariable analysis, and first postRT PSA also became significant (p≤0.029).
Multivariable analysis showed that AIC decreased, indicating improved model performance, each time one of our analysed PSA variables was included. Including all postRT PSA information gave the best model (∆AIC=30, p<0.001).
Interestingly, the first postRT PSA was more prognostic than baseline PSA in all multivariable models (baseline: HR=1, p≤0.014. postRT: HR~4, p≤0.003).
Conclusion
Our results show that PSA dynamics within the first 3-years of RT are prognostic of future BCR.
Further, we demonstrate that including postRT PSA dynamics in predictive models improves model performance, with all postRT PSA variables more prognostic than baseline PSA. These results demonstrate the value of short-term postRT PSA information for prediction of future BCR in men with PCa and provide a mechanism for better targeted follow-up.