uLDR-BT in treating patients with unfavorable intermediate-risk group prostate cancer.
PO-2194
Abstract
uLDR-BT in treating patients with unfavorable intermediate-risk group prostate cancer.
Authors: Adam Kluska1, Adam Chicheł1, Wojciech Burchardt1, Marcin Włodarczyk2, Artur Chyrek1
1Greater Poland Cancer Center, Brachytherapy Department, Poznań, Poland; 2Greater Poland Cancer Center, 3rd Department of Radiotherapy, Poznań, Poland
Show Affiliations
Hide Affiliations
Purpose or Objective
Ultra-low dose rate brachytherapy (uLDR-BT) is effective and widely used as monotherapy for low-risk and favorable intermediate-risk prostate cancer. Treatment with sole uLDR-BT for unfavorable intermediate risk factor (IUR) group prostate cancer patients is not recommended by guidelines due to the lack of solid evidence of its effectiveness. However, in older trials regarding the efficacy of this method in the IUR group, numerous patients were treated with good results. The purpose of this work was to retrospectively assess the effectiveness of uLDR-BT in the IUR group treated in our department.
Material and Methods
We performed a retrospective analysis of 34 IUR prostate cancer patients treated with uLDR-BT between 2015-2018. The local staging was assessed with either MRI or TRUS. The treatment was performed using I-125 seeds, and the total dose prescribed to the clinical target volume (prostate with margin) was 145 Gy. Androgen deprivation therapy (ADT, neoadjuvant or concomitant) was given in 25 cases. All patients had a follow-up in our ambulatory every three months during the first year after treatment and each 4-6 months during the following years. Acute and late toxicities were assessed according to the RTOG scale.
Results
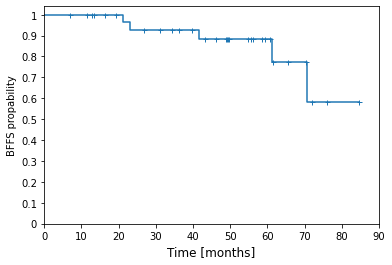
The median follow-up was 49.50 months (IQR 34.29-61.56). Gleason score was 2+ 3 in one case, 3+3 in 7, 3+4 in 13 and 4+3 in 13 cases. Local stage was T1 in 6 cases, T2a in 9, T2b in 8 and T2c in 11 cases. Median time of ADT was 6 months (IQR 6.0-9.0). The initial median PSA level was 10.36 ng/ml (IQR 6.90-14.00), median nadir PSA level was 0.034 ng/ml (IQR 0.004 – 0.099). The estimated 5-year biochemical failure-free survival (BFFS) was 88.5 %. There was no statistically significant impact of ADT on BFFS (p=0.96). Acute grade 3 toxicity (hematuria needing catheterization) occurred in one patient. There was no other acute nor late higher than grade 2 toxicity.
Conclusion
LDR-BT may be an effective option for selected IUR prostate cancer patients. There is a place for prospective studies to fully establish this method's effectiveness in treating IUR prostate cancer.