Congruence between patient-reported outcomes and toxicity of LDR Brachytherapy for prostate cancer
PO-2192
Abstract
Congruence between patient-reported outcomes and toxicity of LDR Brachytherapy for prostate cancer
Authors: LILIA BARDOSCIA1, MARIA ANTONIETTA GILIO2, PAOLA COCUZZA1, MARIAGRAZIA QUATTROCCHI2, MARCELLO MIGNOGNA1
1Radiation Oncology Unit, S. Luca Hospital, Azienda USL Toscana Nord Ovest, Lucca, Italy; 2Medical Physics Department, Azienda USL Toscana Nord Ovest, Lucca, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
To explore the alignment of patient-reported outcomes (PROs) via self-administered questionnaires with clinical assessment of genitourinary (GU) toxicity after Low-Dose-Rate brachytherapy (LDR-BCT) as exclusive treatment for localized prostate cancer (PCa). This study is part of Clinical Outcome of Radiotherapy Treatment in Azienda Toscana Nord-Ovest (CORTATNO) research project funded by Tuscany Region.
Material and Methods
Data from 35 patients treated with I-125 permanent intra-prostatic seeds implantation for low- and favorable intermediate-risk PCa between January 2015 and June 2021 (transrectal ultrasound (US) planning, 145 Gy total target dose) were retrospectively reviewed. Pre-planning US and 1-month post-implant CT dosimetry, GU toxicity outcomes (CT-CAE v5.0) at 1-year follow up (FU), baseline and 1-year post-procedure International Prostate Symptoms Score (IPSS) and International Index of Erectile Function (IIEF) tools were recorded and compared. Clinical, morphofunctional parameters with known or suspected impact on the subject were also collected.
Results
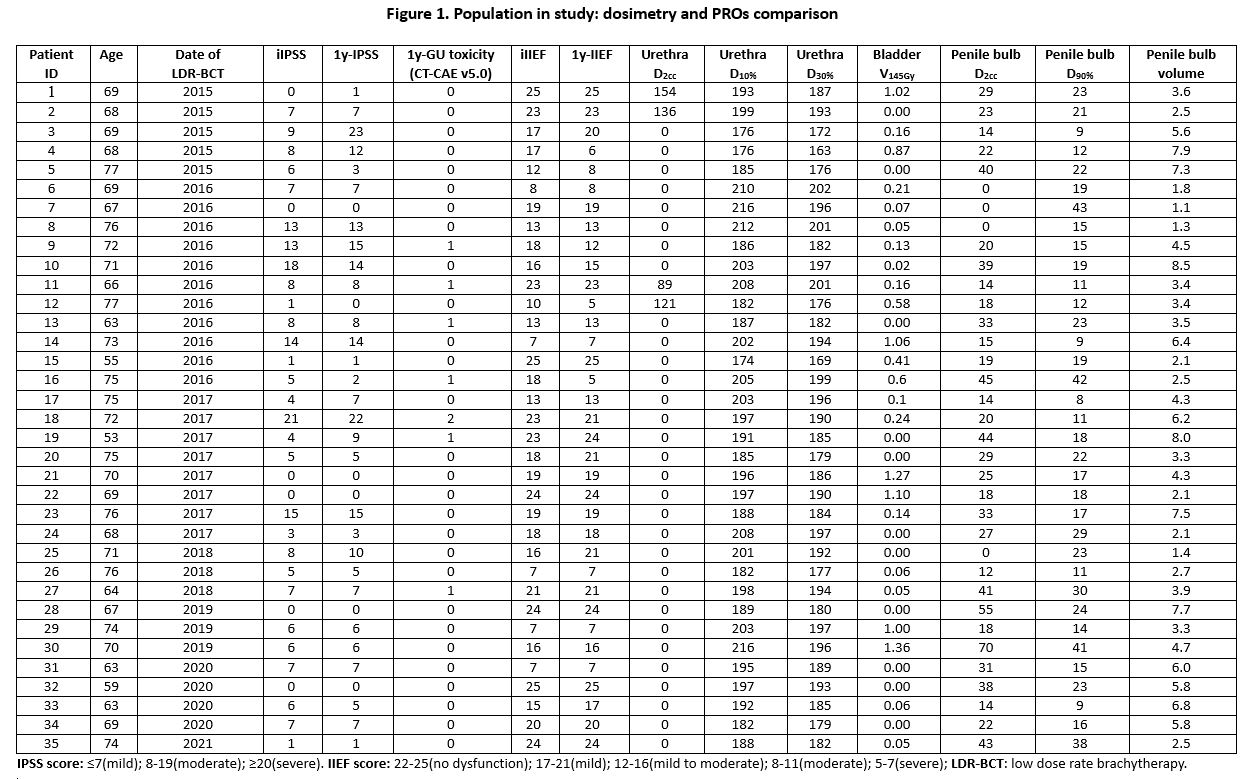 Figure 1 summarizes dosimetric and PROs comparison. At FU interview, 7 (20%) ≥G2 urinary symptoms were found. No patients complained erectile dysfunction. Two (6%) cases of worsened IPSS scores and 6 (17%) changes in IIEF score after LDR-BCT were reported. IPSS results were congruent with the recorded GU toxicity grading in 28 (80%) cases. IPSS score was higher than clinical GU symptoms in 6 (17%) patients, only 1 (3%) patient complained worse IPSS score than clinical relief (p=0.20). Two (6%) patients underwent 3-month androgen cytoreduction before the procedure and, after 1 year, had unaltered IIEF score. Post-implant IPSS worsening significantly correlated with post-planning bladder V145Gy (p=0.02) and slightly correlated with pre-planning urethral D2cc (p=0.045). Both baseline and post-implant IIEF scores correlated with post-planning penile bulb (PB) D2cc (p=0.006 and p=0.003, respectively), while there was a slight correlation between post-procedure IIEF score and post-planning PB D90% (p=0.06). Although not statistically significant, patients complaining sexual impairment showed a slightly higher volume of PB than those without (median 5.4 cc (range 2.5-7.9) versus 3.6 cc (range 1.1-8.5), p=0.81), but no differences between radiation dose to the organs at risk (pre-planning prostatic urethra, post-planning bladder and PB). Evaluation of the radiation dose to neurovascular bundles is ongoing.
Figure 1 summarizes dosimetric and PROs comparison. At FU interview, 7 (20%) ≥G2 urinary symptoms were found. No patients complained erectile dysfunction. Two (6%) cases of worsened IPSS scores and 6 (17%) changes in IIEF score after LDR-BCT were reported. IPSS results were congruent with the recorded GU toxicity grading in 28 (80%) cases. IPSS score was higher than clinical GU symptoms in 6 (17%) patients, only 1 (3%) patient complained worse IPSS score than clinical relief (p=0.20). Two (6%) patients underwent 3-month androgen cytoreduction before the procedure and, after 1 year, had unaltered IIEF score. Post-implant IPSS worsening significantly correlated with post-planning bladder V145Gy (p=0.02) and slightly correlated with pre-planning urethral D2cc (p=0.045). Both baseline and post-implant IIEF scores correlated with post-planning penile bulb (PB) D2cc (p=0.006 and p=0.003, respectively), while there was a slight correlation between post-procedure IIEF score and post-planning PB D90% (p=0.06). Although not statistically significant, patients complaining sexual impairment showed a slightly higher volume of PB than those without (median 5.4 cc (range 2.5-7.9) versus 3.6 cc (range 1.1-8.5), p=0.81), but no differences between radiation dose to the organs at risk (pre-planning prostatic urethra, post-planning bladder and PB). Evaluation of the radiation dose to neurovascular bundles is ongoing.
Conclusion
Despite the low toxicity rates (confirming LDR BCT as a safe therapeutic option for localized PCa), patient-completed tools were substantially in line with clinical evaluation of GU toxicity, but more sensitive and subject to less bias than physician grading about sexual impairment. PROs may be a benefit, owing to fully assess the cost-effectiveness of LDR-BCT and other curative treatments of PCa. Prospective evaluation of our promising findings on wider series is granted.