Mean age was 76 years (range 67-93). 70% were men and 30% women.
The histology was squamous cell in 49%, basal cell in 43% and others in 7%.
According to the 8th TNM-UICC staging, 11% was T1N0M0, 70% was T2N0M0, 11% was T3N0M0, 3% was T3N1M0, 3% T3N2M0 and 2% was T4N0M0.
51% patients had undergone prior surgery on the lesion.
Lesions were located in the ear in 66% cases, in the nose in 9%, in temporal region in 6%, in frontal region in 6%, in the lip in 6%, in the periorbital region in 3%, in the shell in 2% and in the scrotum in 2%.
Dose were delivered according to the following fractionation schemes: 6x7Gy 63%, 9x4.5Gy 25%, 7x7Gy 3%, 5x7Gy 3%, 5x5Gy 3% 25x2Gy 3%.
G1 skin acute toxicity appeared in 40%, G2 in 25% and G3 in 11%. No G4 acute toxicity was observed.
G1 skin chronic toxicity appeared in 32% (13% hypopigmentation, 8% hyperpigmentation, 5% induration, 3% atrophy and 3% fibrosis).
Median OS was 5 years.
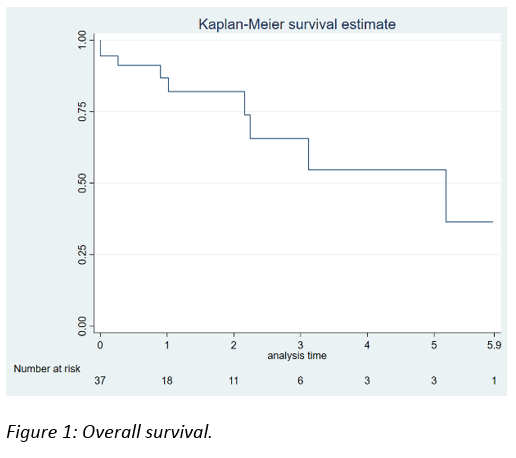
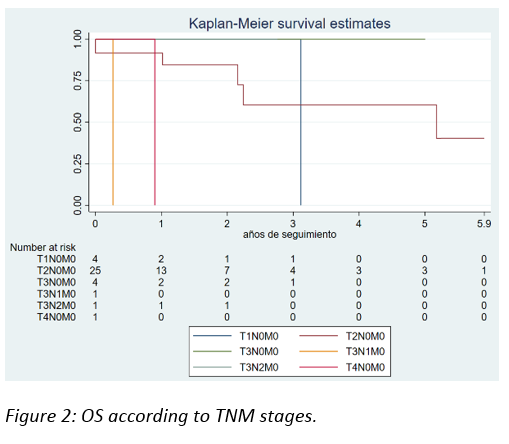
OS according to pathological stage was 3 years in T1, 5 years in T2, less than 2 years in T3 and less than 1 year in T1 stages.
There were statistically significant differences in terms of OS in favor of T1-T2 stages (p=0.061).
At 3 months, complete response (CR) was observed in 77% cases, stable lesion (SL) in 13%, progression (PG) in 7% a partial response (PR) in 3%.
At 6 months, CR was observed in 77% cases, SL in 8%, PG in 12% a PR in 3%.
At 12 months, CR was observed in 67% cases, SL in 13%, PG in 13% a PR in 7%.
Recurrences were observed in 14%, and all of them were treated, 50% with RT and 50% with surgery.
Progression free survival was 81% at 2 years and 69% at 5 years with no differences according to tumor stage (p=0.12).
As October/22, 61% of patients are alive without skin lesion, 14% alive with skin lesion and 25% have died.
The cause of death was unrelated to the skin tumor in 89%.