Commissioning the beam model of apertures in a compact proton system using pencil beam scanning
JUAN MARIA PEREZ MORENO,
Spain
PO-1981
Abstract
Commissioning the beam model of apertures in a compact proton system using pencil beam scanning
Authors: JUAN MARIA PEREZ MORENO1, Juan Antonio Vera Sánchez1, Fernando Cerrón Campoo1, Juan Castro Novais1, Elisabet Canals de las Casas1, Isabel Lorenzo Villanueva1, Morena Sallabanda Hajro1, Marta Montero Feijoo1, Ana de Pablo González1, Raúl Matute Martín1, Raymond Miralbell1, Alejandro Mazal1
1Quirónsalud Protontherapy Center, Radiation Oncology, Pozuelo de Alarcón (Madrid), Spain
Show Affiliations
Hide Affiliations
Purpose or Objective
To validate treatment planning system (TPS) beam model for a snout with brass apertures in proton pencil beam scanning (PBS) beams.
Material and Methods
The Proteus One proton system has a compact nozzle snout holder compatible the Snout S removable accessory, designed to maintain an aperture and a range shifter to modify PBS beams up to 12 cm diameter.
Geometrical, physical and material properties of snout elements can be defined as additional beam line objects for the existing PBS beam model.
A set of 4, 3, 2, 1.5 and 1 cm diameter circular and 2 cm square apertures were used to create validation plans.
First, a 10 cm range, 5 cm modulation and 5 cm diameter circular PBS beam was designed. Absorbed dose at 8 cm depth in water was measured for the resulting fixed spot map using circular apertures using IBA Razor ionization chamber (IC).
Second, absorbed dose, lateral profiles and depth dose distribution were measured for several single PBS beam plans with different values of range and modulation combined with different apertures of the apertures set. Absorbed dose and depth doses were measured with IC while lateral profiles with IC and PTW Octavius 1600XDR.
Finally, dose per beam was measured in an end-to-end test (E2E) defined using Lucy SRS Phantom and 3 beam plan with 2cm aperture.
Results
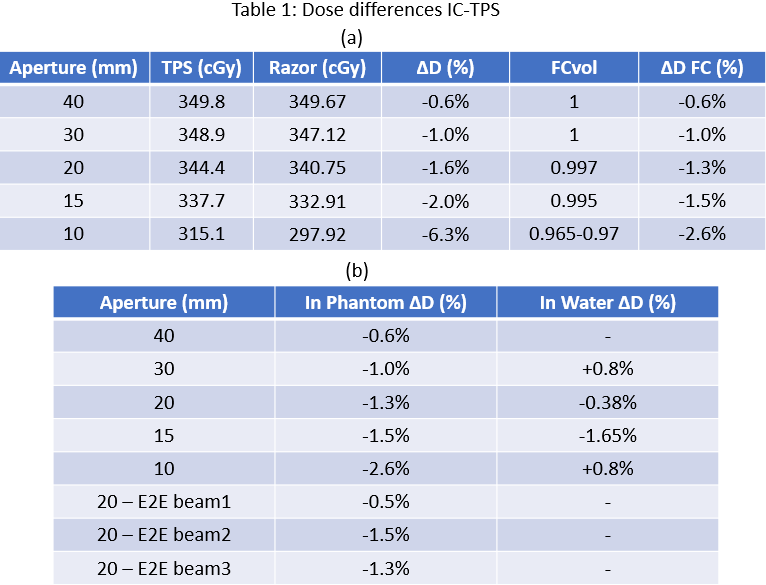
Table 1a shows IC-TPS dose difference for the fixed spot map and varying aperture. Last column refers to differences after applying a volume effect correction factor (FCvol) to IC reading.
Table 1b shows IC-TPS average dose difference for every aperture of the set of different range, modulation and aperture combination, measured in water and in RW3 phantom respectively, as well for the beams of E2E test measured in Lucy phantom.
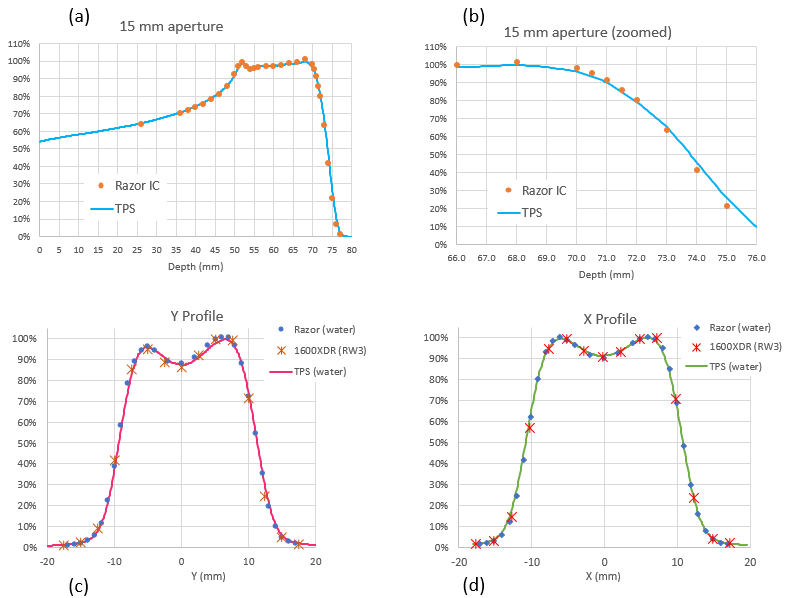
Measured range R90 and R80 agreement with TPS calculation were better than 0.2 mm in all cases. Maximum observed range difference was 0.35 mm for R20 in one plan with 30 mm aperture. Figure 1a-b shows the IC and TPS agreement in a non-homogeneous SOBP.
In Figure 1c-d can be observed X and Y profiles for a heterogeneous dose distribution inside a 20 mm aperture. Maximum deviation in penumbra and field was 0.3 mm for 1600XDR detector, probably due to its limited detector resolution compared with Razor IC.
Conclusion
TPS beam model calculate dose distributions of PBS beams using apertures with good level of accuracy. This finding needs to be confirmed for patient specific apertures with irregular shape.
Absorbed dose measurement is challenging for beam of 1 cm or less. Deeper investigation and test with other small field detectors suitable for proton PBS beams would be recommended to evaluate correction factors that should be used with such small fields.
Apertures in proton beams will increase neutron generation but is expected to be significantly lower than in passive systems, as in PBS protons interact only with block edges. Nevertheless, there is a decrease in lateral penumbra when using apertures, that could mean important dosimetry advantages in some clinical cases.