Rectal tumour position variation: Systematic and random variations by point-based surface evaluation
Dennis Tideman Arp,
Denmark
PO-1932
Abstract
Rectal tumour position variation: Systematic and random variations by point-based surface evaluation
Authors: Dennis Tideman Arp1,2, Ane L Appelt3,4, Martin Skovmos Nielsen1,2, Rasa Mikalone5, Laurids Østergaard Poulsen6,2
1Aalborg University Hospital, Department of Medical Physics, Oncology, Aalborg, Denmark; 2Aalborg University, Department of Clinical Medicine, Aalborg, Denmark; 3University of Leeds, Leeds Institute of Medical Research at St James’s, Leeds, United Kingdom; 4St James’s University Hospital, Leeds Cancer Centre, Leeds, United Kingdom; 5Aalborg University Hospital, Department of Radiology, Aalborg, Denmark; 6Aalborg University Hospital, Department of Oncology, Aalborg, Denmark
Show Affiliations
Hide Affiliations
Purpose or Objective
There is limited data on the interfraction motion of primary rectal tumours, hindering robust PTV margin calculations. We examined in-plane surface variation of the GTV on prospectively collected MRI data using a point-based surface displacement metric, with the aim of estimating systematic and random variations for PTV margin calculation.
Material and Methods
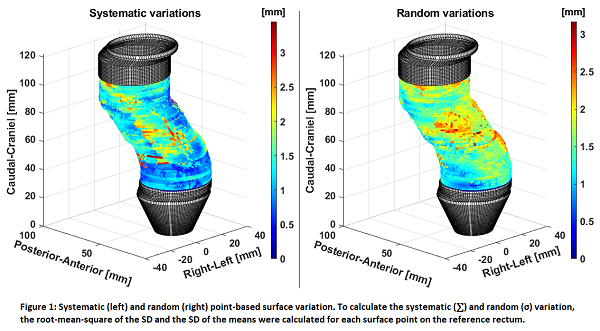
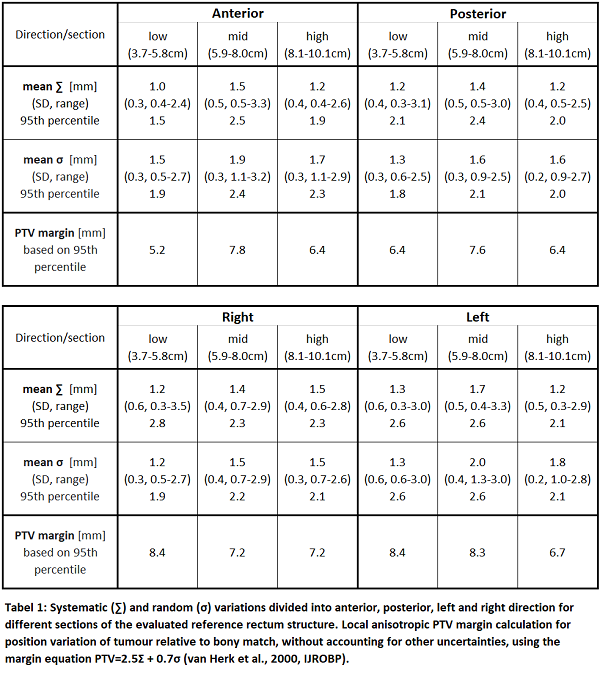
We collected MRI scans before (x3) and during (x3) radiotherapy (RT) in a prospective clinical imaging study (NCT03619668). All patients were treated for locally advanced rectal cancer (T2-4 N0-2) with long course RT (50.4 Gy/28 frac.) and concomitant chemotherapy. The MRI scans (T2-weighted on a Philips Ingenia 3T MRI) were co-registered using a rigid bony match, and GTVs delineated by an experienced oncologist. Each GTV was represented by 5000-23.000 surface points, depending on the size. The within-patient surface variation was calculated using a point-based bidirectional local distance (BLD). For each point position, the distance between the point on the baseline scan and subsequent MRI scans was calculated as a 3D displacement vector. To collate information across patients, a reference rectum structure was created, consisting of 120 equidistant surface points on each slice. Baseline GTV volumes (and their set of displacement vectors) were transferred to the reference geometry using the relative distance from the anal verge and correlated to a point using the BLD metric. Systematic (∑) and random (σ) variation, were calculated for each surface point on the reference rectum (see Figure 1). To evaluate anterior, posterior, and left/right directions, each reference slice was divided into four 90º angle spans (30 points each). To evaluate different regions along the height of the rectum, three sections were defined and analysed separately. Based on ∑ and σ from each section, local anisotropic PTV margins were calculated.
Results
Sixteen patients (7 male, 9 female) were included, with tumors in the lower (13/16) and mid (3/16) rectum. The four top and bottom baseline GTV slices were removed from each patient dataset, to ensure that only in-plane variation was considered. Slices on the reference rectum containing data from less than six patients were excluded. Variation analysis was consequently performed from 3.7 cm to 10.1 cm in the rectum relative to the anal verge, and the sections defined as: low (3.7-5.8cm), mid (5.9-8.0cm), high (8.1-10.1cm). Figure 1 shows ∑ and σ variations on the surface of the reference rectum. Table 1 shows mean variations divided into the sections of the reference rectum, including SD, range and 95th percentile. Local PTV margins based on the 95th percentile variations are provided.
Conclusion
Using prospectively collected MRI scans and innovative point-based surface evaluations, we robustly calculated systematic and random variation for rectal tumours in different sections of the rectum. We calculated corresponding PTV margins to account for position variation when using rigid bony match for positioning.