Robust evaluation of proton therapy within a randomized clinical trial for high-risk prostate cancer
PO-1924
Abstract
Robust evaluation of proton therapy within a randomized clinical trial for high-risk prostate cancer
Authors: Sofie Tilbaek1, Stine Petersen1, Anne Vestergaard1, Liliana Stolarczyk1, Heidi S Rønde1, Tanja Johansen1,2, Lise Bentzen3, Jimmi Søndergaard4, Morten Høyer1, Ludvig Muren1
1Aarhus University Hospital, Danish Center for Particle Therapy, Aarhus, Denmark; 2Rigshospitalet, Department of Oncology, Copenhagen, Denmark; 3Vejle Hospital, University of Southern Denmark, Department of Oncology, Vejle, Denmark; 4Aalborg University Hospital, Department of Oncology, Aalborg, Denmark
Show Affiliations
Hide Affiliations
Purpose or Objective
Robust evaluation (RE) of a proton treatment plan in the planning stage (PS-RE) takes into account geometrical uncertainties in the form of isocenter shifts as well as range uncertainties. Pelvic anatomical variations include shape and position changes that are difficult to quantify and predict. The aim of this study was to compare a delivery stage proton RE (DS-RE) to the PS-RE within the pilot phase of a randomized trial, using clinically observed anatomical variations from weekly control CT scans (cCTs).
Material and Methods
The first three patients included in the pilot phase of a randomized trial (PROstate PROTON Trial 1, NCT05350475) were analyzed. Doses of 78 Gy to the primary target (CTVp - the prostate and involved seminal vesicles) and 56 Gy to the elective target (CTVe - pelvic lymph nodes and the remaining seminal vesicles) were prescribed with simultaneous integrated boost in 39 fractions. A four-field pencil beam scanning configuration of two lateral oblique fields and two posterior oblique fields was used to spare the rectum, bladder and bowel. Internal margins of 4 mm cranio-caudally and 2 mm all other directions accounted for intra- and inter-fractional motion. The PS-RE used 5 mm isotropic isocenter shifts and 3.5% range uncertainty and revealed a span of predicted treatment scenarios from which the original nominal (PS-RE nominal) and worst case (PS-RE worst case) were evaluated for dose/volume measures corresponding to each target and normal tissue constraint. The DS-RE involved 7-8 cCTs per patient which were matched to the pCT using a marker match in line with clinical treatment practice. The original nominal treatment plans were then recalculated on the cCTs including range uncertainty (+/-3.5%) – giving three DS-RE scenarios per cCT. The target and normal tissue constraints from each of the cCT recalculations were compared to the corresponding pCT measures; both for the nominal recalculated plans (DS-REs nominal) and the worst cases (DS-REs worst case).
Results
In the DS-RE, CTVp and CTVe target coverages as well as normal tissue doses were clinically acceptable. However, the PS-RE did not always capture the DS-RE scenarios (Fig. 1). Of the 22 cCTs, 11 had CTVp constraints in the DS-RE outside the range of their respective PS-RE worst case. Similarly, this was the case for 10 of the cCTs when looking at the CTVe constraints. Rectum, bladder and bowel constraints in the DS-RE were worse than their respective PS-RE worst case for 0, 4 and 14 cCTs respectively.
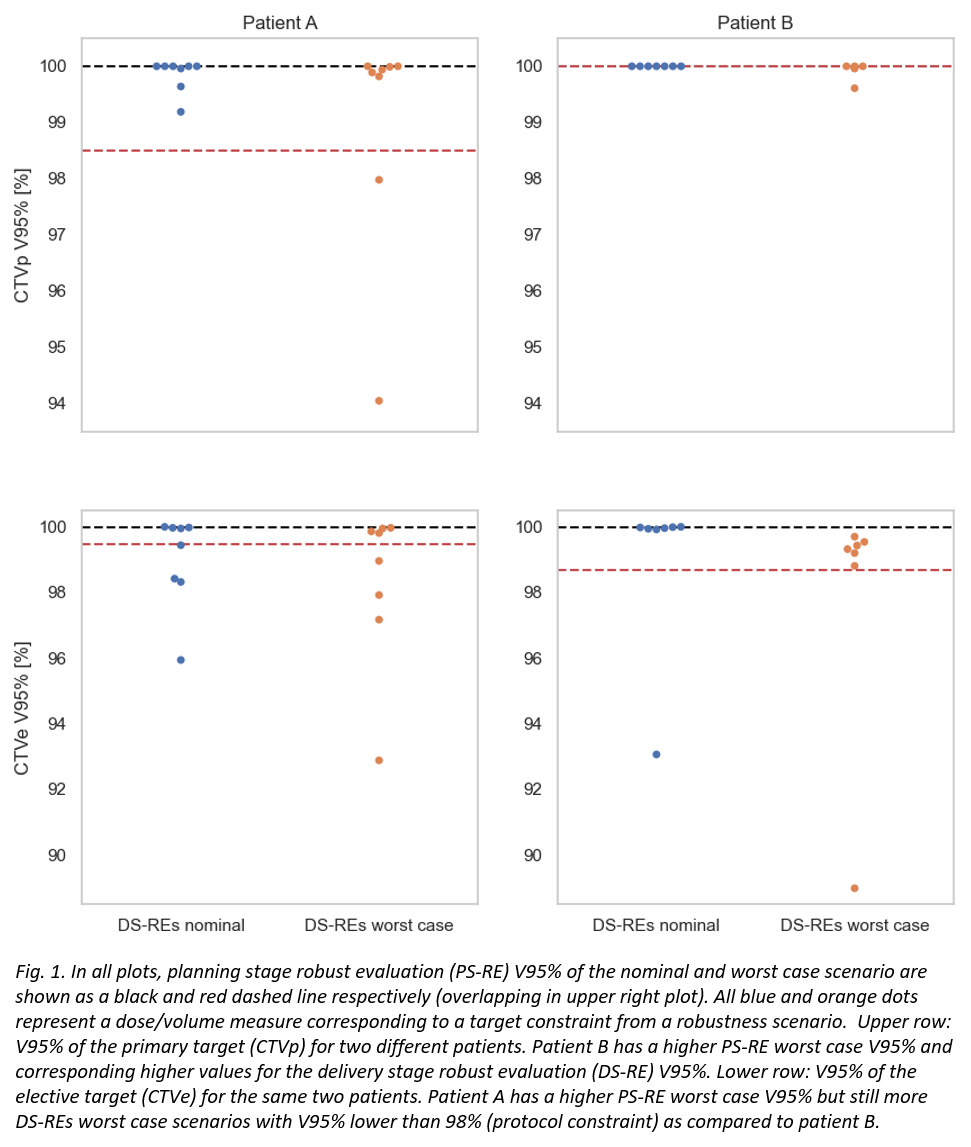
Conclusion
The treatment plans of the first three patients treated in our pilot stage were all clinically accepted both in the planning stage and following DS-RE on the weekly cCTs. The PS-RE did not capture all scenarios found in the DS-RE during the course of therapy. The assessed DS-RE based on cCTs will therefore be used alongside manual clinical evaluation of the CBCTs for offline evaluation of treatment robustness throughout the ongoing pilot phase of our randomized trial.